
The electronic configuration of argon is:
[A] $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}$
[B] $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{2}}$
[C] $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{4}}$
[D] $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}4{{s}^{2}}4{{p}^{6}}$
Answer
583.5k+ views
Hint: The atomic number of argon is 18. We can write the electronic configuration by the help of the atomic number and filling up those electrons in s, p, d and f-orbitals. The number of electrons that can be accommodated in s, p, d and f-orbitals are 2, 6, 10 and 14 respectively.
Complete step by step answer:
We should know that electronic configuration of any atom or molecule gives us the numeric arrangement of electrons around the nucleus.
There are specific notations that we use for writing the configuration of any atom. For writing these notations, we start with the energy orbitals. Practically, there are 4 orbitals s, p, d and f. There is a certain even number of electrons that each orbital can accommodate. s-orbital can accommodate 2 electrons whereas p, d and f-orbitals can accommodate 6, 10 and 14 electrons respectively.
There is a trend each electron follows while filling these orbitals and it is given as-
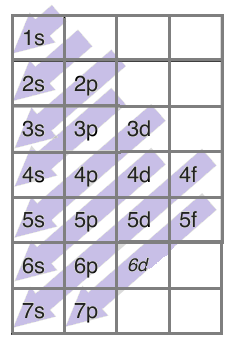
For example, if there are 6 electrons in an element, two electrons will enter 1s orbital first followed by two electrons in 2s-orbital and then the remaining 2 in the 2p-orbital.
Similarly, we can write the electronic configuration for argon. The atomic number of argon is 18 which means it has 18 electrons and 18 protons. We can write the electronic configuration for 18 electrons following the above diagram as-$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}$.
Therefore, the correct answer is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}$.
So, the correct answer is “Option A”.
Note: While writing electronic configuration, we need to consider three rules. They are- Aufbau principle, Hund’s rule and Pauli’s exclusion principle.
According to Aufbau’s principle, the added electron will always occupy the orbital in the lowest possible energy first and then go for higher energy orbitals in a systematic way.
According to Pauli’s exclusion principle, each orbital can hold a maximum of two electrons of opposite spin.
And according to Hund’s rule, while filling a sub-shell each orbital is occupied by electrons in the same spin and when it's filled up then the electrons take the opposite spin.
Complete step by step answer:
We should know that electronic configuration of any atom or molecule gives us the numeric arrangement of electrons around the nucleus.
There are specific notations that we use for writing the configuration of any atom. For writing these notations, we start with the energy orbitals. Practically, there are 4 orbitals s, p, d and f. There is a certain even number of electrons that each orbital can accommodate. s-orbital can accommodate 2 electrons whereas p, d and f-orbitals can accommodate 6, 10 and 14 electrons respectively.
There is a trend each electron follows while filling these orbitals and it is given as-

For example, if there are 6 electrons in an element, two electrons will enter 1s orbital first followed by two electrons in 2s-orbital and then the remaining 2 in the 2p-orbital.
Similarly, we can write the electronic configuration for argon. The atomic number of argon is 18 which means it has 18 electrons and 18 protons. We can write the electronic configuration for 18 electrons following the above diagram as-$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}$.
Therefore, the correct answer is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}$.
So, the correct answer is “Option A”.
Note: While writing electronic configuration, we need to consider three rules. They are- Aufbau principle, Hund’s rule and Pauli’s exclusion principle.
According to Aufbau’s principle, the added electron will always occupy the orbital in the lowest possible energy first and then go for higher energy orbitals in a systematic way.
According to Pauli’s exclusion principle, each orbital can hold a maximum of two electrons of opposite spin.
And according to Hund’s rule, while filling a sub-shell each orbital is occupied by electrons in the same spin and when it's filled up then the electrons take the opposite spin.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




