
The electronic configuration of nitrogen is 2, 5. How many electrons in the outer shell of the nitrogen atom are not involved in the formation of a nitrogen molecule?
Answer
534k+ views
Hint: While the formation of the nitrogen molecule, each nitrogen atom is deficient of 3 electrons to complete their octet. So, each atom will share 3 electrons to form the bond and the rest of the electrons will not be involved in bond formation.
Complete step by step solution: The nitrogen belongs to group 15 of the periodic table and its electronic configuration is –
\[\text{N}\to \text{1}{{\text{s}}^{2}}2{{\text{s}}^{2}}2{{\text{p}}^{3}}\text{ or }\left[ \text{He} \right]2{{\text{s}}^{2}}2{{\text{p}}^{3}}\]
We can see that the inner 1s – orbital is fully filled, so it will not take place in bonding. The outermost orbital of nitrogen contains a total of 5 electrons \[\left( 2{{\text{s}}^{2}}2{{\text{p}}^{3}} \right)\] . The nitrogen needs 3 more electrons to complete its octet.
Now, when two atoms of nitrogen approach each other, they both equally share three electrons each and form a triple covalent bond to complete their octet as shown below.

The electronic configuration of the system will look like this:
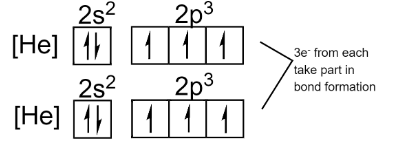
Thus, we can see that out of 5 valence electrons only 3 are involved in the bonding during the formation of a nitrogen molecule. So, the remaining 2 electrons remain intact in each nitrogen atom.
Hence, two electrons in the outer shell of each nitrogen atom are not involved in the formation of a nitrogen molecule.
Note: The nitrogen forms a covalent bond with another nitrogen atom by sharing its 3 valence electrons. It cannot completely accept or donate electrons to form an ionic bond due to the presence of stable half-filled p-orbital in its valence shell.
Complete step by step solution: The nitrogen belongs to group 15 of the periodic table and its electronic configuration is –
\[\text{N}\to \text{1}{{\text{s}}^{2}}2{{\text{s}}^{2}}2{{\text{p}}^{3}}\text{ or }\left[ \text{He} \right]2{{\text{s}}^{2}}2{{\text{p}}^{3}}\]
We can see that the inner 1s – orbital is fully filled, so it will not take place in bonding. The outermost orbital of nitrogen contains a total of 5 electrons \[\left( 2{{\text{s}}^{2}}2{{\text{p}}^{3}} \right)\] . The nitrogen needs 3 more electrons to complete its octet.
Now, when two atoms of nitrogen approach each other, they both equally share three electrons each and form a triple covalent bond to complete their octet as shown below.

The electronic configuration of the system will look like this:

Thus, we can see that out of 5 valence electrons only 3 are involved in the bonding during the formation of a nitrogen molecule. So, the remaining 2 electrons remain intact in each nitrogen atom.
Hence, two electrons in the outer shell of each nitrogen atom are not involved in the formation of a nitrogen molecule.
Note: The nitrogen forms a covalent bond with another nitrogen atom by sharing its 3 valence electrons. It cannot completely accept or donate electrons to form an ionic bond due to the presence of stable half-filled p-orbital in its valence shell.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




