
The electrons present in the outermost shell of an atom are known as:
(A) Protons
(B) Neutrons
(C) Octet
(D) Valence electrons
Answer
562.8k+ views
Hint: An atom consists of three basic particles which are protons, electrons, and neutrons. The atom is the smallest particle in which protons and neutrons are present in the center and electrons revolve around it. Electrons revolve around the nucleus in certain definite paths called orbits or shells.
Complete answer:
The structure of an atom is as shown below:
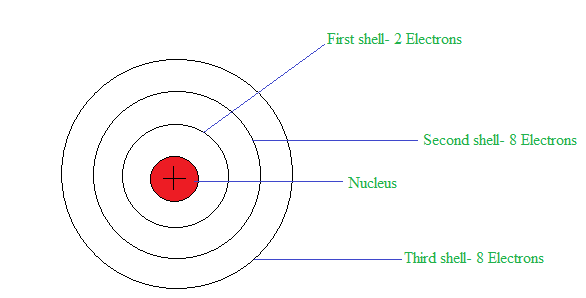
As stated in the Bohr’s model of an atom, electrons travel in a definite circular orbit also known as shells. In addition to this, the last orbit is known as the outermost shell. If the outermost shell is completely filled with electrons then it is a noble gas.
If the outermost shell is partially filled with electrons then it becomes a valence shell. The electrons present in the valence shell are known as valence electrons.
The valence electrons which are present in the outermost shell participate in the compound formation. An atom achieves stability either by donating valence electrons or by accepting extra electrons from another atom. This is done in order to gain the stable configuration or basically to complete the octet.
Thus, the electrons present in the outermost shell of an atom are known as valence electrons.
So, the correct choice is (D).
Note: The atoms don’t achieve stability only by donating or accepting the valence electrons. Sometimes stability is achieved from various types of bonds by sharing the electrons. A chemical bond is basically the force of attraction between any two atoms in a molecule required to maintain stability. These various types of bonds are covalent bond, triple bond, hydrogen bonding, ionic bond, polar covalent bond, metallic bond, etc. In a group there are the same number of electrons in the outermost shell.
Complete answer:
The structure of an atom is as shown below:

As stated in the Bohr’s model of an atom, electrons travel in a definite circular orbit also known as shells. In addition to this, the last orbit is known as the outermost shell. If the outermost shell is completely filled with electrons then it is a noble gas.
If the outermost shell is partially filled with electrons then it becomes a valence shell. The electrons present in the valence shell are known as valence electrons.
The valence electrons which are present in the outermost shell participate in the compound formation. An atom achieves stability either by donating valence electrons or by accepting extra electrons from another atom. This is done in order to gain the stable configuration or basically to complete the octet.
Thus, the electrons present in the outermost shell of an atom are known as valence electrons.
So, the correct choice is (D).
Note: The atoms don’t achieve stability only by donating or accepting the valence electrons. Sometimes stability is achieved from various types of bonds by sharing the electrons. A chemical bond is basically the force of attraction between any two atoms in a molecule required to maintain stability. These various types of bonds are covalent bond, triple bond, hydrogen bonding, ionic bond, polar covalent bond, metallic bond, etc. In a group there are the same number of electrons in the outermost shell.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




