
The energy level diagram below represents the different energy states for a particular atom. What is the energy of a photon which is associated with the shortest possible wavelength emitted by this atom?
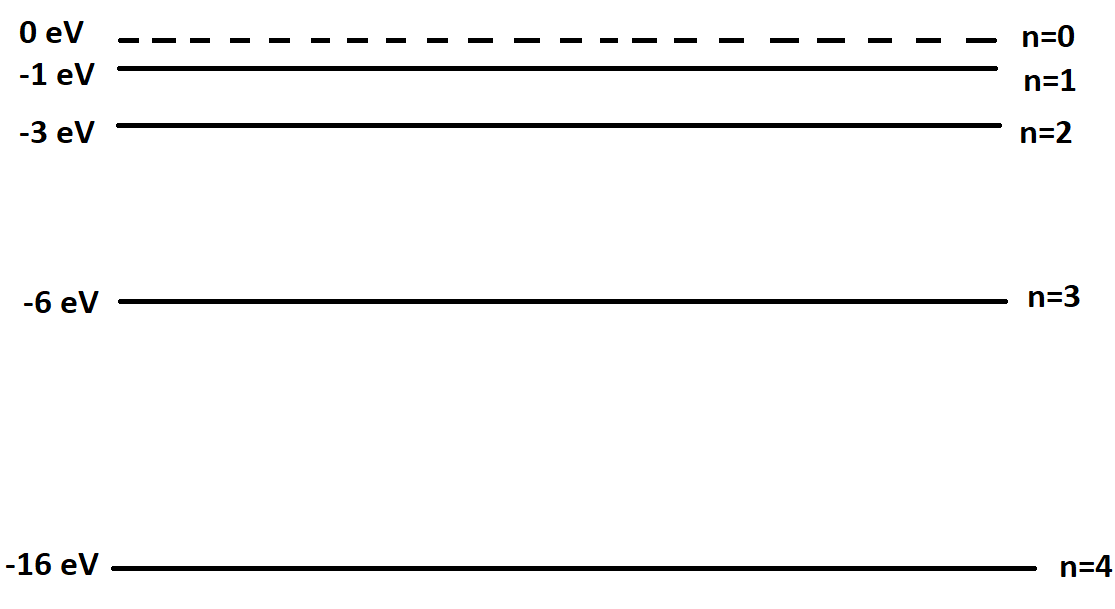
\[{\text{A}}{\text{. 16 eV}}\]
\[{\text{B}}{\text{. 8 eV}}\]
\[{\text{C}}{\text{. 13 eV}}\]
\[{\text{D}}{\text{. 15 eV}}\]
\[{\text{E}}{\text{. 1 eV}}\]

Answer
478.2k+ views
Hint: The energy of the photon is inversely proportional to the wavelength and thus, directly proportional to the frequency. This is because wavelength and frequency are inversely proportional to each other. As the wavelength increases, the energy of the photon decreases.
Formula used: \[E = \dfrac{{hc}}{\lambda }\]
Where, E is the energy of the photon,
$h$ is Planck's constant,
$c$ is the velocity of light in vacuum,
\[\lambda \] is the wavelength.
Complete step by step answer:
The energy of the photon using the Planck-Einstein equation is as follows:
\[E = \dfrac{{hc}}{\lambda }\]
Therefore energy emitted during transition
\[ \Rightarrow E \propto \dfrac{1}{\lambda }\]
Now using the fact that maximum wavelength is for the minimum emission of energy and minimum wavelength is for maximum emission of energy. So the transition that emits radiation of least energy will have the maximum wavelength and the radiation with maximum energy will have minimum wavelength.
Therefore photons of shortest wavelength will emit maximum energy. From the options given we can say $16eV$ is maximum.
Therefore, Option (A) is correct.
Note: Tiny particles having no charge and no resting mass are known as photons. The photons are emitted by charged particles, radioactive decay, etc. photons always move at the speed of light in vacuum.
Photons can be destroyed as well as created. When electromagnetic waves are emitted by the source, photons are created. When photons hit with matter, they either absorb or transfer the energy to the atoms and molecules. The creation and destruction of photons conserves energy and momentum.
Formula used: \[E = \dfrac{{hc}}{\lambda }\]
Where, E is the energy of the photon,
$h$ is Planck's constant,
$c$ is the velocity of light in vacuum,
\[\lambda \] is the wavelength.
Complete step by step answer:
The energy of the photon using the Planck-Einstein equation is as follows:
\[E = \dfrac{{hc}}{\lambda }\]
Therefore energy emitted during transition
\[ \Rightarrow E \propto \dfrac{1}{\lambda }\]
Now using the fact that maximum wavelength is for the minimum emission of energy and minimum wavelength is for maximum emission of energy. So the transition that emits radiation of least energy will have the maximum wavelength and the radiation with maximum energy will have minimum wavelength.
Therefore photons of shortest wavelength will emit maximum energy. From the options given we can say $16eV$ is maximum.
Therefore, Option (A) is correct.
Note: Tiny particles having no charge and no resting mass are known as photons. The photons are emitted by charged particles, radioactive decay, etc. photons always move at the speed of light in vacuum.
Photons can be destroyed as well as created. When electromagnetic waves are emitted by the source, photons are created. When photons hit with matter, they either absorb or transfer the energy to the atoms and molecules. The creation and destruction of photons conserves energy and momentum.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




