
The excess pressure inside an air bubble of radius r just below the surface of water is ${{p}_{1}}$. The excess pressure inside a drop of the same radius just outside the surface is ${{p}_{2}}$. If T is the surface tension, then:
A). ${{p}_{1}}=2{{p}_{2}}$
B). ${{p}_{1}}={{p}_{2}}$
C). ${{p}_{2}}=2{{p}_{1}}$
D). ${{p}_{2}}=0,{{p}_{1}}\ne 0$
Answer
525.8k+ views
Hint: For a liquid drop we have only one surface. For an air bubble we have two surfaces. Define the mathematical expression for the excess pressure inside an air bubble and inside a liquid drop using the concept of surface tension. Take their ratio to find the answer to this question.
Complete step by step answer:
Consider an air bubble of radius r and surface tension T. we have two free surfaces in an air bubble. Due to the surface tension, the molecules of the surface experience a net inward force towards the centre and normal to the surface.
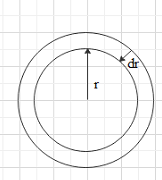
Let the outside pressure is ${{p}_{o}}$ and the inside pressure is ${{p}_{i}}$ .
So, the excess pressure will be $p={{p}_{i}}-{{p}_{o}}$
Due to the excess pressure inside the bubble will expand. Let the bubble expand by $dr$ .
So, the work done by the excess pressure will be.
$\begin{align}
& \text{work done = force }\times \text{ displacement} \\
& \text{work done = excess pressure }\times \text{ surface area }\times \text{ displacement} \\
& \text{work done = p}\times \text{4}\pi {{\text{r}}^{2}}\times dr \\
\end{align}$
Increase in potential energy is given by
$\begin{align}
& =\text{ surface tension }\times \text{ increase in surface area} \\
& =\text{ }T\times \left[ 2\left\{ 4\pi {{\left( r+dr \right)}^{2}}-4\pi {{r}^{2}} \right\} \right] \\
& =T\times 2\times 4\pi \times 2rdr \\
\end{align}$
Comparing these two equations we get that,
$\begin{align}
& p\times 4\pi {{r}^{2}}\times dr=T\times 2\times 4\pi \times 2rdr \\
& p=\dfrac{4T}{r} \\
\end{align}$
So, the excess pressure for an air bubble will be ${{p}_{1}}=\dfrac{4T}{r}$
Again, for a liquid drop we only have one free surface. Following the same procedure, we can find that the excess pressure for liquid drop is ${{p}_{2}}=\dfrac{2T}{r}$
Taking the ratio of these two quantities,
$\begin{align}
& \dfrac{{{p}_{1}}}{{{p}_{2}}}=\dfrac{\dfrac{4T}{r}}{\dfrac{2T}{r}}=2 \\
& {{p}_{1}}=2{{p}_{2}} \\
\end{align}$
The correct option is (A)
Note: While solving numerical related to this always remember that if we consider the a soap bubble or air bubble the excess pressure will be given as $\dfrac{4T}{r}$ and if we consider a liquid drop such as water drop the excess pressure will be given as $\dfrac{2T}{r}$.
Complete step by step answer:
Consider an air bubble of radius r and surface tension T. we have two free surfaces in an air bubble. Due to the surface tension, the molecules of the surface experience a net inward force towards the centre and normal to the surface.

Let the outside pressure is ${{p}_{o}}$ and the inside pressure is ${{p}_{i}}$ .
So, the excess pressure will be $p={{p}_{i}}-{{p}_{o}}$
Due to the excess pressure inside the bubble will expand. Let the bubble expand by $dr$ .
So, the work done by the excess pressure will be.
$\begin{align}
& \text{work done = force }\times \text{ displacement} \\
& \text{work done = excess pressure }\times \text{ surface area }\times \text{ displacement} \\
& \text{work done = p}\times \text{4}\pi {{\text{r}}^{2}}\times dr \\
\end{align}$
Increase in potential energy is given by
$\begin{align}
& =\text{ surface tension }\times \text{ increase in surface area} \\
& =\text{ }T\times \left[ 2\left\{ 4\pi {{\left( r+dr \right)}^{2}}-4\pi {{r}^{2}} \right\} \right] \\
& =T\times 2\times 4\pi \times 2rdr \\
\end{align}$
Comparing these two equations we get that,
$\begin{align}
& p\times 4\pi {{r}^{2}}\times dr=T\times 2\times 4\pi \times 2rdr \\
& p=\dfrac{4T}{r} \\
\end{align}$
So, the excess pressure for an air bubble will be ${{p}_{1}}=\dfrac{4T}{r}$
Again, for a liquid drop we only have one free surface. Following the same procedure, we can find that the excess pressure for liquid drop is ${{p}_{2}}=\dfrac{2T}{r}$
Taking the ratio of these two quantities,
$\begin{align}
& \dfrac{{{p}_{1}}}{{{p}_{2}}}=\dfrac{\dfrac{4T}{r}}{\dfrac{2T}{r}}=2 \\
& {{p}_{1}}=2{{p}_{2}} \\
\end{align}$
The correct option is (A)
Note: While solving numerical related to this always remember that if we consider the a soap bubble or air bubble the excess pressure will be given as $\dfrac{4T}{r}$ and if we consider a liquid drop such as water drop the excess pressure will be given as $\dfrac{2T}{r}$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a labelled diagram of the human heart and label class 11 biology CBSE

What is 1s 2s 2p 3s 3p class 11 chemistry CBSE




