
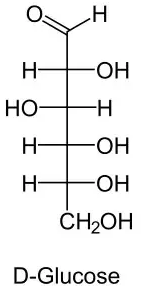
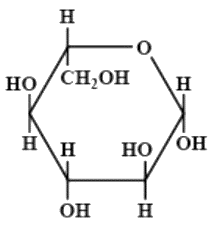
The Fischer presentation of D-glucose is given above:
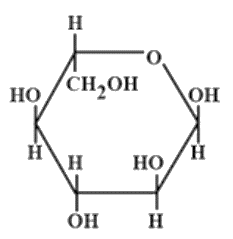
The correct structures of $ \beta - L $ glucopyranose is/are:
(A)
(B)
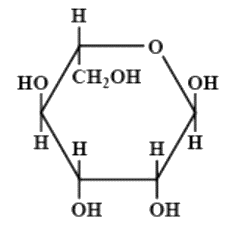
(C)
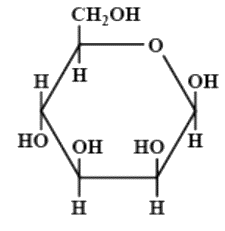
(D)





Answer
548.7k+ views
Hint: To answer this question, you should recall the concept of cyclisation of sugars. We know that the reducing carbohydrates possess anomeric carbon which acts as a chiral centre for the cyclization of the molecules. The cyclization of the carbohydrate molecules forms two different Structural isomers -1) Pyranose 2) furanose.
Complete step by step solution:
Since glucose is an optically active molecule due to chirality, therefore it can show optical isomers which are known as Enantiomers known as $ L\left( - \right) $ glucose and $ D\left( - \right) $ glucose. Here the +ve sign and -ve sign indicates its optical rotation, i.e. dextrorotatory and laevorotatory.
On the other hand, D & L are not related with their optical rotation but they indicate their configurations. The D and L configuration is based on the configuration of a glyceraldehyde molecule which contains one chiral atom. If the $ OH $ group of glyceraldehyde molecules lies towards the right side, it is known as D- configuration otherwise it will be L-configuration.
The resulting structures will be:
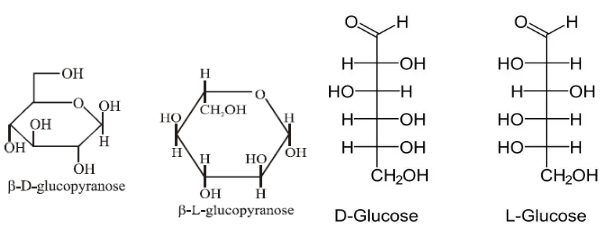
Hence, the correct option is option D.
Note:
You should know that if you have seen drawings of sugars before, you might not have noticed the carbonyl. The reason is that carbonyl is usually "masked" as a hemiacetal structure. The hemiacetal structure is generated when a hydroxyl group along the carbon chain reaches back and bonds to the electrophilic carbonyl carbon. We can see from the structure of fructose that the keto group is present on the second carbon. Now two possibilities for cyclization exist: If attacked by the $ {\text{ - C}}{{\text{H}}_{\text{2}}}{\text{OH}} $ on the 6th carbon it will generate pyranose (6-membered) ring and second that, attacked by the $ {\text{ - CH}}\left( {{\text{OH}}} \right){\text{ - }} $ group on the 5th carbon it will result in a furanose. In general, we can conclude that pyranose rings are more stable than furanose rings, because there are more conformational isomeric structures available for pyranose vs. furanose.
Complete step by step solution:
Since glucose is an optically active molecule due to chirality, therefore it can show optical isomers which are known as Enantiomers known as $ L\left( - \right) $ glucose and $ D\left( - \right) $ glucose. Here the +ve sign and -ve sign indicates its optical rotation, i.e. dextrorotatory and laevorotatory.
On the other hand, D & L are not related with their optical rotation but they indicate their configurations. The D and L configuration is based on the configuration of a glyceraldehyde molecule which contains one chiral atom. If the $ OH $ group of glyceraldehyde molecules lies towards the right side, it is known as D- configuration otherwise it will be L-configuration.
The resulting structures will be:

Hence, the correct option is option D.
Note:
You should know that if you have seen drawings of sugars before, you might not have noticed the carbonyl. The reason is that carbonyl is usually "masked" as a hemiacetal structure. The hemiacetal structure is generated when a hydroxyl group along the carbon chain reaches back and bonds to the electrophilic carbonyl carbon. We can see from the structure of fructose that the keto group is present on the second carbon. Now two possibilities for cyclization exist: If attacked by the $ {\text{ - C}}{{\text{H}}_{\text{2}}}{\text{OH}} $ on the 6th carbon it will generate pyranose (6-membered) ring and second that, attacked by the $ {\text{ - CH}}\left( {{\text{OH}}} \right){\text{ - }} $ group on the 5th carbon it will result in a furanose. In general, we can conclude that pyranose rings are more stable than furanose rings, because there are more conformational isomeric structures available for pyranose vs. furanose.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




