
The formula for potassium permanganate is:
$
A{\text{ }}{{\text{K}}_2}{\text{Mn}}{{\text{O}}_4} \\
B{\text{ KMn}}{{\text{O}}_4} \\
C{\text{ }}{{\text{K}}_2}M{n_2}{O_4} \\
D{\text{ KM}}{{\text{n}}_2}{O_4} \\
$
Answer
611.7k+ views
Hint- In order to solve this question, we must know the formula of potassium permanganate. Then we will compare all the four options to get the required answer.
Complete answer:
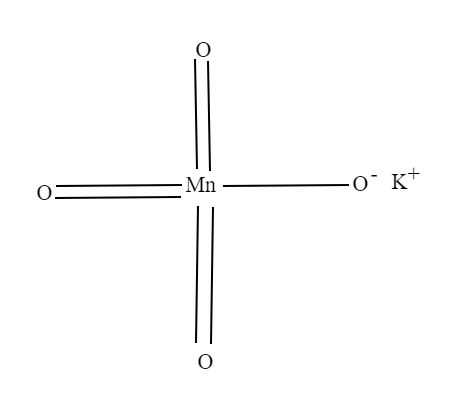
Potassium permanganate is an inorganic compound with the chemical formula$KMn{O_4}$. It is composed of ${K^ + }$and$Mn{O_4}^ - $.
Potassium permanganate is produced industrially from manganese dioxide, which also occurs as the mineral pyrolusite. The $Mn{O_2}$is fused with potassium hydroxide and heated in air or with another source of oxygen, like potassium nitrate or potassium chlorate.
The process gives potassium manganate-
$2Mn{O_2} + 4KOH + {O_2} \to 2{K_2}Mn{O_4} + 2{H_2}O$
The potassium manganate is then converted into permanganate by electrolytic oxidation in alkaline media-
$2{K_2}Mn{O_4} + 2{H_2}O \to 2KMn{O_4} + 2KOH + {H_2}$
In a neutral medium, $Mn{O_2}$forms a brown deposit.
$2KMn{O_4} + 3{K_2}S{O_3} + {H_2}O \to 3{K_2}S{O_4} + 2Mn{O_2} + 2KOH$
Potassium permanganate can oxidize aldehydes to form carboxylic acids.
Potassium permanganate oxidizes alcohols to form carbonyls.
Potassium permanganate cam also oxidize carbon atoms that have triple bonds to form diones.
Potassium permanganate oxidizes sugar to acids by breaking the carbon skeleton at double bonds.
${{\text{K}}_2}{\text{Mn}}{{\text{O}}_4}$is potassium manganate is the inorganic compound. It is of green color salt used in the industrial synthesis of potassium permanganate, a common chemical. It is widely used in laboratories as a strong oxidizing agent and for cleaning wounds, infections.
Therefore, we conclude that ${\text{KMn}}{{\text{O}}_4}$is the chemical formula of Potassium permanganate.
Hence, option B is correct.
Note- While solving this question, we must know the names and chemical formula of all compounds like here we are asked about potassium permanganate. Also we must know the physical and chemical properties of compounds to solve all related questions.

Complete answer:
Potassium permanganate is an inorganic compound with the chemical formula$KMn{O_4}$. It is composed of ${K^ + }$and$Mn{O_4}^ - $.
Potassium permanganate is produced industrially from manganese dioxide, which also occurs as the mineral pyrolusite. The $Mn{O_2}$is fused with potassium hydroxide and heated in air or with another source of oxygen, like potassium nitrate or potassium chlorate.
The process gives potassium manganate-
$2Mn{O_2} + 4KOH + {O_2} \to 2{K_2}Mn{O_4} + 2{H_2}O$
The potassium manganate is then converted into permanganate by electrolytic oxidation in alkaline media-
$2{K_2}Mn{O_4} + 2{H_2}O \to 2KMn{O_4} + 2KOH + {H_2}$
In a neutral medium, $Mn{O_2}$forms a brown deposit.
$2KMn{O_4} + 3{K_2}S{O_3} + {H_2}O \to 3{K_2}S{O_4} + 2Mn{O_2} + 2KOH$
Potassium permanganate can oxidize aldehydes to form carboxylic acids.
Potassium permanganate oxidizes alcohols to form carbonyls.
Potassium permanganate cam also oxidize carbon atoms that have triple bonds to form diones.
Potassium permanganate oxidizes sugar to acids by breaking the carbon skeleton at double bonds.
${{\text{K}}_2}{\text{Mn}}{{\text{O}}_4}$is potassium manganate is the inorganic compound. It is of green color salt used in the industrial synthesis of potassium permanganate, a common chemical. It is widely used in laboratories as a strong oxidizing agent and for cleaning wounds, infections.
Therefore, we conclude that ${\text{KMn}}{{\text{O}}_4}$is the chemical formula of Potassium permanganate.
Hence, option B is correct.
Note- While solving this question, we must know the names and chemical formula of all compounds like here we are asked about potassium permanganate. Also we must know the physical and chemical properties of compounds to solve all related questions.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

The Equation xxx + 2 is Satisfied when x is Equal to Class 10 Maths

Which Country is Called "The Land of Festivals"?

What is Contraception List its four different methods class 10 biology CBSE




