
The formula of Aluminium oxide is \[A{{l}_{2}}{{O}_{3}}\]. Find the valencies of Aluminium and Oxygen.
Answer
610.8k+ views
- Hint: We know that the atomic number of Aluminium is 13 and the atomic number of Oxygen is 8. Using the criss-cross method we can determine the valency of Aluminium and Oxygen in Aluminium oxide or \[A{{l}_{2}}{{O}_{3}}\].
Complete step by step answer:
We know that according to Bohr’s atomic model, the number of electrons that can be present on the different shells is determined by the formula \[2({{n}^{2}})\], where n denotes the number of the shell.
Since the atomic number of Aluminium is 13, using the formula \[2({{n}^{2}})\] we get,
In the first shell, the number of electrons is \[2\times {{1}^{2}}=2\] electrons.
In the second shell, the number of electrons is \[2\times {{2}^{2}}=8\] electrons.
In the third shell, the number of valence electrons is = 3 electrons.
Since \[2+8+3=13\] electrons, the atomic number of Aluminium is 13.
Now, the atomic number of Oxygen is 8. Using the formula \[2({{n}^{2}})\] we get,
In the first shell, the number of electrons is \[2\times {{1}^{2}}=2\] electrons
In the second shell, the number of valence electrons = 6 electrons.
Since \[2+6=8\] electrons, the atomic number of Oxygen is 8.
Therefore, to attain the octet configuration, Aluminium loses 3 electrons, and Oxygen gains 2 electrons.
Now, we have to write the formula of Aluminium Oxide using the criss-cross method.
We know that in the criss-cross method the numerical value of each of the ion charges is crossed over to become the subscript of the other ion.
The charge of Aluminium is +3 and the charge of Oxygen is -2.
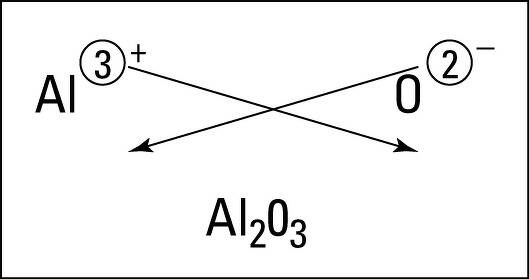
Therefore, we can conclude that the valency of Aluminium in \[A{{l}_{2}}{{O}_{3}}\] is 3 and the valency of Oxygen in \[A{{l}_{2}}{{O}_{3}}\] is 2.
Note: Aluminium loses the 3 valence electrons to gain the noble gas configuration of Neon, which is 2,8.
Similarly, Oxygen gains 2 valence electrons to gain the noble gas configuration of Neon, that is 2,8.
Complete step by step answer:
We know that according to Bohr’s atomic model, the number of electrons that can be present on the different shells is determined by the formula \[2({{n}^{2}})\], where n denotes the number of the shell.
Since the atomic number of Aluminium is 13, using the formula \[2({{n}^{2}})\] we get,
In the first shell, the number of electrons is \[2\times {{1}^{2}}=2\] electrons.
In the second shell, the number of electrons is \[2\times {{2}^{2}}=8\] electrons.
In the third shell, the number of valence electrons is = 3 electrons.
Since \[2+8+3=13\] electrons, the atomic number of Aluminium is 13.
Now, the atomic number of Oxygen is 8. Using the formula \[2({{n}^{2}})\] we get,
In the first shell, the number of electrons is \[2\times {{1}^{2}}=2\] electrons
In the second shell, the number of valence electrons = 6 electrons.
Since \[2+6=8\] electrons, the atomic number of Oxygen is 8.
Therefore, to attain the octet configuration, Aluminium loses 3 electrons, and Oxygen gains 2 electrons.
Now, we have to write the formula of Aluminium Oxide using the criss-cross method.
We know that in the criss-cross method the numerical value of each of the ion charges is crossed over to become the subscript of the other ion.
The charge of Aluminium is +3 and the charge of Oxygen is -2.

Therefore, we can conclude that the valency of Aluminium in \[A{{l}_{2}}{{O}_{3}}\] is 3 and the valency of Oxygen in \[A{{l}_{2}}{{O}_{3}}\] is 2.
Note: Aluminium loses the 3 valence electrons to gain the noble gas configuration of Neon, which is 2,8.
Similarly, Oxygen gains 2 valence electrons to gain the noble gas configuration of Neon, that is 2,8.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




