
The gas leaked from a storage tank of the Union Carbide plant in Bhopal gas tragedy was:
a- Methylamine
b- Ammonia
c- Phosgene
d- Methyl isocyanate
Answer
577.5k+ views
Hint: The gas leaked from a storage tank of the Union Carbide plant in Bhopal gas tragedy was methyl isocyanate (\[C{H_3}NCO\]).
Complete answer:
Methyl isocyanate (MIC) is an organic compound with the molecular formula \[C{H_3}NCO\] and it is very toxic.
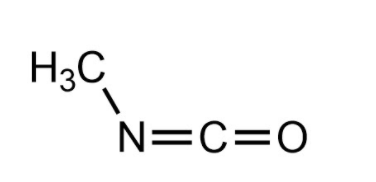
Methyl isocyanate is a poisonous, lachrymatory (tearing agent), colorless, flammable liquid with a sharp pungent odor and It is soluble in water.
Methyl isocyanate is usually prepared by the reaction of mono methylamine with phosgene at high temperatures. For production on a large scale, it is advantageous to combine these reactants at higher temperatures in the gas phase. By this mixture, one mole of methyl isocyanate and two moles of hydrogen chloride is formed.
The toxic effect of this compound appeared in the 1984 Bhopal disaster when around 42,000 kilograms of methyl isocyanate and other gases were released from the underground reservoirs of the Union Carbide India Limited (UCIL) factory. This incident happened on December 3, 1984. This chemical killed about 3,500 people immediately and 15,000 more over the next several years.
Hence, the answer is an option (d) i.e. methyl isocyanate.
Note:
Remember that the gas was methyl isocyanate, not methyl cyanide. Methyl cyanide doesn’t show instant toxic effects. Methyl isocyanate exposure can cause pulmonary edema, bronchospasm, or an electrolyte imbalance in the human body.
Complete answer:
Methyl isocyanate (MIC) is an organic compound with the molecular formula \[C{H_3}NCO\] and it is very toxic.
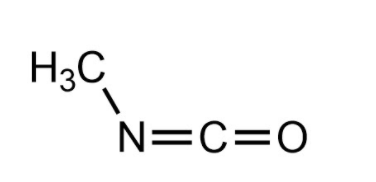
Methyl isocyanate is a poisonous, lachrymatory (tearing agent), colorless, flammable liquid with a sharp pungent odor and It is soluble in water.
Methyl isocyanate is usually prepared by the reaction of mono methylamine with phosgene at high temperatures. For production on a large scale, it is advantageous to combine these reactants at higher temperatures in the gas phase. By this mixture, one mole of methyl isocyanate and two moles of hydrogen chloride is formed.
The toxic effect of this compound appeared in the 1984 Bhopal disaster when around 42,000 kilograms of methyl isocyanate and other gases were released from the underground reservoirs of the Union Carbide India Limited (UCIL) factory. This incident happened on December 3, 1984. This chemical killed about 3,500 people immediately and 15,000 more over the next several years.
Hence, the answer is an option (d) i.e. methyl isocyanate.
Note:
Remember that the gas was methyl isocyanate, not methyl cyanide. Methyl cyanide doesn’t show instant toxic effects. Methyl isocyanate exposure can cause pulmonary edema, bronchospasm, or an electrolyte imbalance in the human body.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




