
The geometry of $Xe{{O}_{3}}$ is ________.
(A) linear
(B) planar
(C) pyramidal
(D) T-shaped
Answer
588.6k+ views
Hint: Start by writing electronic configuration in Xenon oxide. Calculate the number of electron pairs in the given molecule, $Xe{{O}_{3}}$. Find out the hybridization of $Xe{{O}_{3}}$ and draw the structure accordingly.
Complete step by step answer:
- Xenon belongs to the group of inert gases and it has the atomic number 54.
- Its electronic configuration is,
\[Xe=1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{10}}4{{p}^{6}}5{{s}^{2}}4{{d}^{10}}5{{p}^{6}}\]
- Oxygen has the atomic number 8. Its electronic configuration is,
\[O=1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}\]
- For Xenon trioxide, $Xe{{O}_{3}}$, let’s calculate the number of electron pairs.
- Xenon has 8 valence electrons and oxygen has 6 valence electrons.
- Therefore, $8+\left( 3\times 6 \right)=\dfrac{26}{2}=13$ electron pairs. So, $Xe{{O}_{3}}$ has 13 electron pairs present in it.
- Let’s draw the Lewis structure for $Xe{{O}_{3}}$.
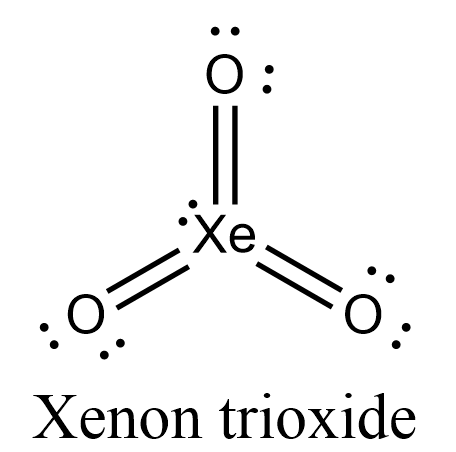
- In this structure, we can see that all 13 electron pairs are present either as bond-pairs or as lone-pairs.
- Let’s take a look at its hybridization now. Xenon has vacant 5d orbitals so, when xenon gets excited its fifth orbital configuration will become $5{{s}^{2}}5{{p}^{3}}5{{d}^{3}}$. 5s and 5p orbitals will undergo $s{{p}^{3}}$ hybridization.
- Therefore, Xenon in xenon trioxide will have three $s{{p}^{3}}$ hybrid orbitals, containing three unpaired electrons and one lone pair of electrons, and unhybridized 5d orbitals having three unpaired electrons.
- Oxygen will undergo $s{{p}^{2}}$ hybridization and will have two $s{{p}^{2}}$ hybridized orbitals having one lone pair of electrons each and one $s{{p}^{2}}$ hybridized orbital containing one unpaired electron. It will have one unhybridized 2p orbital having one electron.
- The three $s{{p}^{3}}$ orbitals of xenon having three unpaired electrons will axially overlap with three $s{{p}^{2}}$ orbitals of three individual oxygen atoms to form three $\sigma -bonds$. The three 5d orbitals will laterally overlap with three 2p unhybridized orbitals of three oxygen atoms to form three $\pi -bonds$.
- Now, each oxygen atom has two lone pairs of electrons and a xenon atom has one lone pair of electrons. Xenon and oxygen are doubly bonded to each other.
- Due to the presence of lone pair on central atom Xenon, there will be lone pair-bond pair repulsion in the molecule. So, the trigonal planar geometry will be distorted to form pyramidal geometry.
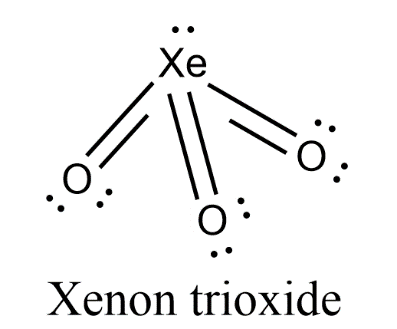
- Therefore, the geometry of $Xe{{O}_{3}}$ is pyramidal.
So, the correct answer is “Option C”.
Note: Remember when there is a lone pair present on the central atom, there will be lone pair- bond pair repulsion between the central atom and the other atoms linked to it. This will lead to distortion of geometry. Xenon trioxide is an unstable compound and it is a very powerful oxidizing agent.
Complete step by step answer:
- Xenon belongs to the group of inert gases and it has the atomic number 54.
- Its electronic configuration is,
\[Xe=1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{10}}4{{p}^{6}}5{{s}^{2}}4{{d}^{10}}5{{p}^{6}}\]
- Oxygen has the atomic number 8. Its electronic configuration is,
\[O=1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}\]
- For Xenon trioxide, $Xe{{O}_{3}}$, let’s calculate the number of electron pairs.
- Xenon has 8 valence electrons and oxygen has 6 valence electrons.
- Therefore, $8+\left( 3\times 6 \right)=\dfrac{26}{2}=13$ electron pairs. So, $Xe{{O}_{3}}$ has 13 electron pairs present in it.
- Let’s draw the Lewis structure for $Xe{{O}_{3}}$.

- In this structure, we can see that all 13 electron pairs are present either as bond-pairs or as lone-pairs.
- Let’s take a look at its hybridization now. Xenon has vacant 5d orbitals so, when xenon gets excited its fifth orbital configuration will become $5{{s}^{2}}5{{p}^{3}}5{{d}^{3}}$. 5s and 5p orbitals will undergo $s{{p}^{3}}$ hybridization.
- Therefore, Xenon in xenon trioxide will have three $s{{p}^{3}}$ hybrid orbitals, containing three unpaired electrons and one lone pair of electrons, and unhybridized 5d orbitals having three unpaired electrons.
- Oxygen will undergo $s{{p}^{2}}$ hybridization and will have two $s{{p}^{2}}$ hybridized orbitals having one lone pair of electrons each and one $s{{p}^{2}}$ hybridized orbital containing one unpaired electron. It will have one unhybridized 2p orbital having one electron.
- The three $s{{p}^{3}}$ orbitals of xenon having three unpaired electrons will axially overlap with three $s{{p}^{2}}$ orbitals of three individual oxygen atoms to form three $\sigma -bonds$. The three 5d orbitals will laterally overlap with three 2p unhybridized orbitals of three oxygen atoms to form three $\pi -bonds$.
- Now, each oxygen atom has two lone pairs of electrons and a xenon atom has one lone pair of electrons. Xenon and oxygen are doubly bonded to each other.
- Due to the presence of lone pair on central atom Xenon, there will be lone pair-bond pair repulsion in the molecule. So, the trigonal planar geometry will be distorted to form pyramidal geometry.

- Therefore, the geometry of $Xe{{O}_{3}}$ is pyramidal.
So, the correct answer is “Option C”.
Note: Remember when there is a lone pair present on the central atom, there will be lone pair- bond pair repulsion between the central atom and the other atoms linked to it. This will lead to distortion of geometry. Xenon trioxide is an unstable compound and it is a very powerful oxidizing agent.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




