
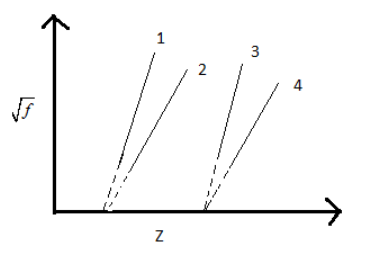
The given graph shows the variation of $\sqrt{f}$ vs Z for characteristics X-rays. Lines 1, 2, 3, 4 shown in the graph corresponds to any one of ${{K}_{\alpha }}$, ${{K}_{\beta }}$, ${{L}_{\alpha }}$ and ${{L}_{\beta }}$, then line ${{L}_{\beta }}$ is represented by (f=frequency, Z =atomic number)
(A)Line 1
(B)Line 2
(C)Line 3
(D)Line 4

Answer
582.9k+ views
Hint: The four lines represent the 4 lines of characteristic X ray given as ${{K}_{\alpha }}$, ${{K}_{\beta }}$, ${{L}_{\alpha }}$ and ${{L}_{\beta }}$. To deduce which line represents which we must understand the energy values of these lines and try to establish a relationship among them.
Complete answer:
We know,
In characteristic X rays, the K series of lines have higher energy values than the L series of lines ....................... (1)
Moreover, we have
The $\beta $ lines are more energetic in comparison to the $\alpha $ lines. .................. (2)
We know,
Energy is directly proportional to the frequency. So more is the energy, more is the frequency which is represented by the y-axis. ................... (3)
Using the information given by (1), (2) and (3), we can arrange the lines in ascending or descending order as per our choice and identify the ${{L}_{\beta }}$ line.
In ascending order of frequency, the lines can be arranged as
${{L}_{\alpha }}<{{L}_{\beta }}<{{K}_{\alpha }}<{{K}_{\beta }}$
From the series in ascending order, we can deduce that the ${{L}_{\beta }}$line is the 3rd line.
So the correct answer is (C) Line 3.
Additional Information:
The study of atomic spectra which is based on some set of Selection Rules is a very important source which provides us the knowledge of atoms and molecules. Characteristic X rays are emitted when the outer-shell electrons fill a vacancy in the inner shell of an atom, releasing X-rays in a pattern which is the characteristic of the element.
Note:
In this problem we must know certain facts. We must understand that the K set of lines are more energetic than the L set of lines and also that the $\beta $ lines are more energetic than the $\alpha $ . Also we must understand the relationship between energy and frequency and relate the three sets of information into finding the ${{L}_{\beta }}$ line.
Complete answer:
We know,
In characteristic X rays, the K series of lines have higher energy values than the L series of lines ....................... (1)
Moreover, we have
The $\beta $ lines are more energetic in comparison to the $\alpha $ lines. .................. (2)
We know,
Energy is directly proportional to the frequency. So more is the energy, more is the frequency which is represented by the y-axis. ................... (3)
Using the information given by (1), (2) and (3), we can arrange the lines in ascending or descending order as per our choice and identify the ${{L}_{\beta }}$ line.
In ascending order of frequency, the lines can be arranged as
${{L}_{\alpha }}<{{L}_{\beta }}<{{K}_{\alpha }}<{{K}_{\beta }}$
From the series in ascending order, we can deduce that the ${{L}_{\beta }}$line is the 3rd line.
So the correct answer is (C) Line 3.
Additional Information:
The study of atomic spectra which is based on some set of Selection Rules is a very important source which provides us the knowledge of atoms and molecules. Characteristic X rays are emitted when the outer-shell electrons fill a vacancy in the inner shell of an atom, releasing X-rays in a pattern which is the characteristic of the element.
Note:
In this problem we must know certain facts. We must understand that the K set of lines are more energetic than the L set of lines and also that the $\beta $ lines are more energetic than the $\alpha $ . Also we must understand the relationship between energy and frequency and relate the three sets of information into finding the ${{L}_{\beta }}$ line.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




