
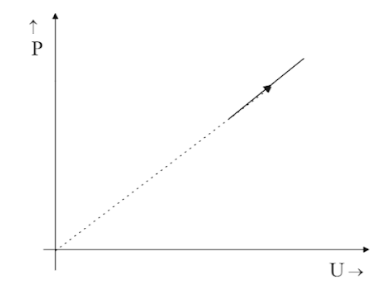
The given P-U graphs show the variation of internal energy of an ideal gas with increase in pressure. Which of the following pressure volume graphs is equivalent to this graph?
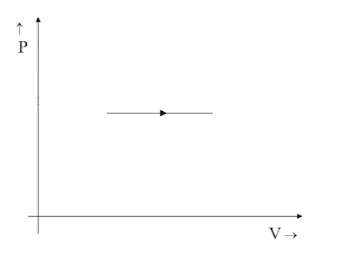
(A)
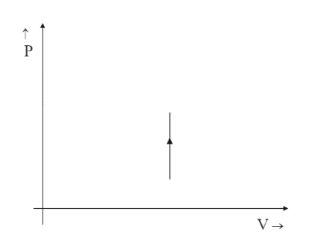
(B)
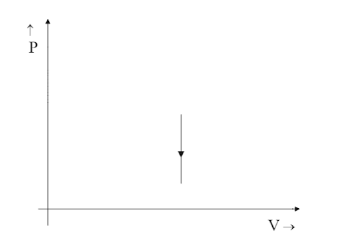
(C)
(D)





Answer
564.3k+ views
Hint: The P-U graph is a straight line passing through origin, thus making an angle of $ 45^\circ $ with both the X-axis and Y-axis. This clearly states that $ P \propto U $ . Also, Temperature and Volume are related by the ideal gas equation, $ PV = nRT $ .
Formulas Used: We use the formulas $ PV = nRT $ where $ P $ is the pressure of the gas, $ V $ is the volume of the gas, $ n $ is the number of moles of the gas, $ R $ is the gas constant, and $ T $ is the temperature of the gas. We would also be using the formula $ U = \dfrac{3}{2}RT $ where $ U $ is the internal energy of the system.
Complete Step by Step solution
Internal energy is one of the thermodynamic properties of an ideal gas. The internal energy is basically the sum of all the energies an ideal gas possesses. Usually internal energy is only the sum of Kinetic energy and Potential energy. But in case of an ideal gas, the particle of the ideal gas does not interact with each other, hence it does not possess any potential energy. Also, this also suggests that the internal energy of an ideal gas is nothing but the sum of the kinetic energy of its particle.
We also know that according to kinetic molecular theory the temperature of a gas is directly proportional to the average of its kinetic energy. Thus proposing the relation $ U = \dfrac{3}{2}RT $ , thus stating that the temperature of the ideal gas $ T $ and the internal energy of the ideal gas $ U $ is also directly proportional to each other.
$ \Rightarrow T \propto U $
The graph we have is a P-U graph that depicts that the Pressure $ P $ and internal energy $ U $ are proportional to each other directly. $ P \propto U $ .
Since both $ P $ and $ T $ are directly proportional to $ U $ $ \Rightarrow P \propto T $ .
We know that $ PV = nRT $
$ \Rightarrow T = \dfrac{{PV}}{{nR}} $ .
So, substituting $ T = \dfrac{{PV}}{{nR}} $ in $ P \propto {\rm T} $ we get,
$ P \propto \dfrac{{PV}}{{nR}} $ $ \Rightarrow 1 \propto \dfrac{V}{{nR}} $
So $ V $ does not vary with the variables and hence remains constant, since the relation should be calculated for unit mole $ (n = 1) $ and $ R $ is a gas constant.
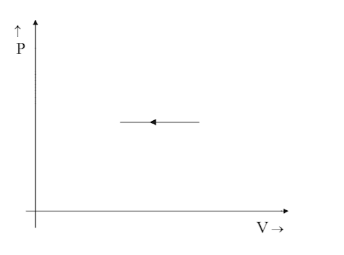
Thus $ V $ remains constant, which is a line that is straight and parallel to the Y-axis in the positive direction.
Hence option B is correct.
Note
Since the relation would be $ 1 \propto V $ the graph would point up in the positive direction stating it is increasing with increasing values of the Y-axis and not the opposite. That is why option C is ruled out even though the graph is a straight line parallel to Y-axis.
Formulas Used: We use the formulas $ PV = nRT $ where $ P $ is the pressure of the gas, $ V $ is the volume of the gas, $ n $ is the number of moles of the gas, $ R $ is the gas constant, and $ T $ is the temperature of the gas. We would also be using the formula $ U = \dfrac{3}{2}RT $ where $ U $ is the internal energy of the system.
Complete Step by Step solution
Internal energy is one of the thermodynamic properties of an ideal gas. The internal energy is basically the sum of all the energies an ideal gas possesses. Usually internal energy is only the sum of Kinetic energy and Potential energy. But in case of an ideal gas, the particle of the ideal gas does not interact with each other, hence it does not possess any potential energy. Also, this also suggests that the internal energy of an ideal gas is nothing but the sum of the kinetic energy of its particle.
We also know that according to kinetic molecular theory the temperature of a gas is directly proportional to the average of its kinetic energy. Thus proposing the relation $ U = \dfrac{3}{2}RT $ , thus stating that the temperature of the ideal gas $ T $ and the internal energy of the ideal gas $ U $ is also directly proportional to each other.
$ \Rightarrow T \propto U $
The graph we have is a P-U graph that depicts that the Pressure $ P $ and internal energy $ U $ are proportional to each other directly. $ P \propto U $ .
Since both $ P $ and $ T $ are directly proportional to $ U $ $ \Rightarrow P \propto T $ .
We know that $ PV = nRT $
$ \Rightarrow T = \dfrac{{PV}}{{nR}} $ .
So, substituting $ T = \dfrac{{PV}}{{nR}} $ in $ P \propto {\rm T} $ we get,
$ P \propto \dfrac{{PV}}{{nR}} $ $ \Rightarrow 1 \propto \dfrac{V}{{nR}} $
So $ V $ does not vary with the variables and hence remains constant, since the relation should be calculated for unit mole $ (n = 1) $ and $ R $ is a gas constant.
Thus $ V $ remains constant, which is a line that is straight and parallel to the Y-axis in the positive direction.
Hence option B is correct.
Note
Since the relation would be $ 1 \propto V $ the graph would point up in the positive direction stating it is increasing with increasing values of the Y-axis and not the opposite. That is why option C is ruled out even though the graph is a straight line parallel to Y-axis.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




