
The ground state electron configuration of the cobalt atom is characterized by which of the following?
I. Partially filled 3d orbital
II. The presence of unpaired electrons
III. All electrons are paired
A. I only
B. II only
C. I and II only
D. I and III only
E. I, II and III
Answer
562.5k+ views
Hint:The atomic number of cobalt is 27. Thus, the electronic configuration will be . The valence electrons in the cobalt atom is 9. The 3d orbital of cobalt contains 7 electrons and 4s orbital contains 2 electrons.
Complete step by step answer:
The cobalt is the chemical element present in the d-block of the periodic table in period 4 and group 9. The cobalt is symbolized by Co. The atomic number of cobalt is 27. The electronic configuration of cobalt is $[Ar]3{d^7}4{s^2}$. The valence electrons are those electrons which are present in the outermost electronic configuration of the atom. The valence electrons in the cobalt atom is 9. The 3d orbital of cobalt contains 7 electrons and 4s orbital contains 2 electrons.
The molecular orbital structure of the ground state of the cobalt atom is shown below.
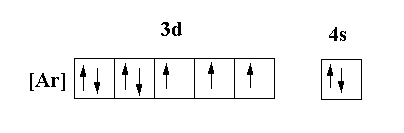
In the ground state of the cobalt atom, the 3d orbital contains 7 electrons and 4s orbital contains 2 electrons. In 3d orbital two orbitals are fully occupied and three orbital contain unpaired electrons and 4s orbital contain fully occupied orbital.
So, there is a partially filled 3d orbital and presence of unpaired electrons. Thus, statements (I) and (II) are correct.
Therefore, the correct option is C.
Note:
The d orbital contains a total 5 orbital which can occupy a total of 10 electrons, each orbital occupies 2 electrons with opposite spin. In s orbital only one orbital is present which can contain 2 electrons with opposite spin.
Complete step by step answer:
The cobalt is the chemical element present in the d-block of the periodic table in period 4 and group 9. The cobalt is symbolized by Co. The atomic number of cobalt is 27. The electronic configuration of cobalt is $[Ar]3{d^7}4{s^2}$. The valence electrons are those electrons which are present in the outermost electronic configuration of the atom. The valence electrons in the cobalt atom is 9. The 3d orbital of cobalt contains 7 electrons and 4s orbital contains 2 electrons.
The molecular orbital structure of the ground state of the cobalt atom is shown below.

In the ground state of the cobalt atom, the 3d orbital contains 7 electrons and 4s orbital contains 2 electrons. In 3d orbital two orbitals are fully occupied and three orbital contain unpaired electrons and 4s orbital contain fully occupied orbital.
So, there is a partially filled 3d orbital and presence of unpaired electrons. Thus, statements (I) and (II) are correct.
Therefore, the correct option is C.
Note:
The d orbital contains a total 5 orbital which can occupy a total of 10 electrons, each orbital occupies 2 electrons with opposite spin. In s orbital only one orbital is present which can contain 2 electrons with opposite spin.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




