
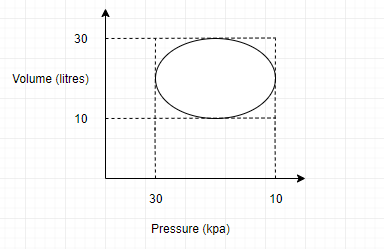
The heat energy absorbed by a system in going through a cyclic process shown in figure is:
A. ${{10}^{7}}\pi J$
B. ${{10}^{6}}\pi J$
C. ${{10}^{2}}\pi J$
D. ${{10}^{4}}\pi J$

Answer
582.6k+ views
Hint: The graph of a cyclic process is given in the question. Here, the system starts the process and further returns to the same thermodynamic state. To find out the heat energy that is absorbed, we have to find out the work done in the entire process from the given P-V graph.
Formulas used:
\[\Delta Q=\Delta U+\Delta W\]: First law of thermodynamics, where \[\Delta Q\] is the heat absorbed, \[\Delta U\] is the internal energy and \[\Delta W\] is the work done by the system.
Complete step by step answer:
Since the process is cyclic and it returns to the initial state, the change in internal energy doesn’t take place, i.e. \[\Delta U=0\]
On applying the first law of thermodynamics, i.e. \[\Delta Q=\Delta U+\Delta W\],
We get the value of\[\Delta Q=\Delta W\], as \[\Delta U=0\]
Work done by the thermodynamic system is the enclosed area of the graph.
Hence, \[\Delta Q=\Delta W=\pi ab\](area of the ellipse)
Here, a is the semi major axis and b is the semi minor axis of the ellipse.
\[a=b=10\](Given)
Therefore \[\pi ab=\]\[{{10}^{2}}\pi J\]=work done= total heat absorbed by the system.
Hence, option C is the right answer among the given options.
Note:Cyclic process is the underlying principle of the functioning of engines. When the graph goes clockwise, we can say that the value of W is positive and this represents a heat engine and if it goes counter clockwise, the value of W is negative and it will act as a heat pump.
Formulas used:
\[\Delta Q=\Delta U+\Delta W\]: First law of thermodynamics, where \[\Delta Q\] is the heat absorbed, \[\Delta U\] is the internal energy and \[\Delta W\] is the work done by the system.
Complete step by step answer:
Since the process is cyclic and it returns to the initial state, the change in internal energy doesn’t take place, i.e. \[\Delta U=0\]
On applying the first law of thermodynamics, i.e. \[\Delta Q=\Delta U+\Delta W\],
We get the value of\[\Delta Q=\Delta W\], as \[\Delta U=0\]
Work done by the thermodynamic system is the enclosed area of the graph.
Hence, \[\Delta Q=\Delta W=\pi ab\](area of the ellipse)
Here, a is the semi major axis and b is the semi minor axis of the ellipse.
\[a=b=10\](Given)
Therefore \[\pi ab=\]\[{{10}^{2}}\pi J\]=work done= total heat absorbed by the system.
Hence, option C is the right answer among the given options.
Note:Cyclic process is the underlying principle of the functioning of engines. When the graph goes clockwise, we can say that the value of W is positive and this represents a heat engine and if it goes counter clockwise, the value of W is negative and it will act as a heat pump.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




