
The height of a liquid in a fine capillary tube:
A. Increases with an increase in the density of a liquid.
B. Decreases with a decrease in the diameter of the tube.
C. Decreases with an increase in the surface tension.
D. Increase as the effective value of acceleration due to gravity is decreased.
Answer
526.3k+ views
Hint: Capillary tube is any thin tube inserted in a liquid to rise the level of liquid inside the tube naturally. When a capillary tube is immersed in liquid, due to the surface tension of liquid, it applies some force on the interface of the tube. Due to this reason, liquid climbs some height in the capillary tube, called capillary rise. Hence some liquid will enter the tube and hence the net weight of the tube will increase.
Formula used:
$h = \dfrac{2Tcos\theta}{r\rho g}$.
Complete answer:
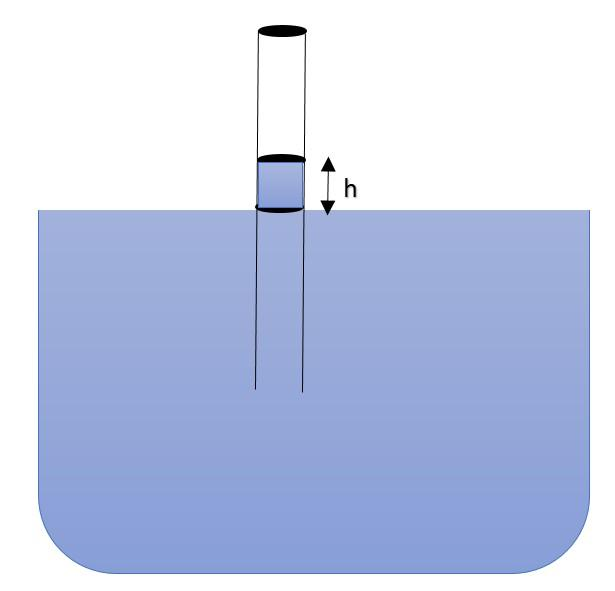
As per the expression:$h = \dfrac{2Tcos\theta}{r\rho g}$, we can say that height climbed by the liquid depends upon the surface tension (T) of the liquid, the angle of contact ($\theta$) between surface of tube and liquid, radius of tube (r), density of liquid ($\rho$) and net value of acceleration due to gravity (g).
It could be clearly seen that if the effective value of acceleration due to gravity (g) is decreased, the net value of height of column (h) will increase.
So, the correct answer is “Option D”.
Surface tension: It is the property of liquid by the virtue of which the surface of the liquid acts as a stretched membrane. In other words, the surface wants to minimize its area and hence when a body is immersed in a liquid, liquid exerts a force on the touching boundary of the liquid and the body.
Note:
This expression of height could be calculated by equating the weight of water rose with $2\pi Tr$, which is the force exerted by the liquid on the container liquid interface so that the force in turn gets applied to the liquid and it gets lifted.
Formula used:
$h = \dfrac{2Tcos\theta}{r\rho g}$.
Complete answer:

As per the expression:$h = \dfrac{2Tcos\theta}{r\rho g}$, we can say that height climbed by the liquid depends upon the surface tension (T) of the liquid, the angle of contact ($\theta$) between surface of tube and liquid, radius of tube (r), density of liquid ($\rho$) and net value of acceleration due to gravity (g).
It could be clearly seen that if the effective value of acceleration due to gravity (g) is decreased, the net value of height of column (h) will increase.
So, the correct answer is “Option D”.
Surface tension: It is the property of liquid by the virtue of which the surface of the liquid acts as a stretched membrane. In other words, the surface wants to minimize its area and hence when a body is immersed in a liquid, liquid exerts a force on the touching boundary of the liquid and the body.
Note:
This expression of height could be calculated by equating the weight of water rose with $2\pi Tr$, which is the force exerted by the liquid on the container liquid interface so that the force in turn gets applied to the liquid and it gets lifted.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




