
The hybridisation of atomic orbitals of nitrogen in $N{ O }_{ 2 }^{ + }$, $N{ O }_{ 3 }^{ - }$ and $N{ H }_{ 4 }^{ + }$ are:
(A) sp, $ { sp }^{ 3 }$ and $ { sp }^{ 2 }$ respectively
(B) sp, $ { sp }^{ 2 }$ and $ { sp }^{ 3 }$ respectively
(C) $ { sp }^{ 2 }$, sp and $ { sp }^{ 3 }$ respectively
(D) $ { sp }^{ 2 }$, $ { sp }^{ 3 }$ and sp respectively
Answer
598.5k+ views
Hint: Here we need to determine the hybridization of the central atom i.e. nitrogen atom. For that we first need to determine the Lewis structures of the molecules. Always try to draw the Lewis structures such that the octet of each atom in the structure is complete.
Complete step by step solution:
Hybridization is achieved through linear combination of atomic orbitals and the new orbitals are called hybrid orbitals. In order to determine the hybridization of an atom in a molecule we should follow the following steps:
-First you have to determine the Lewis structure of the molecule.
-Now assign the regions of electron density around the atom using VSEPR theory for predicting the shape of the molecule (single bonds, multiple bonds, radicals, and lone pairs each will count as one region).
-Now we can determine the set of hybridized orbitals corresponding to the geometry determined using VSEPR theory.
Let us start with $N{ O }_{ 2 }^{ + }$:
The Lewis structure of $N{ O }_{ 2 }^{ + }$ is:
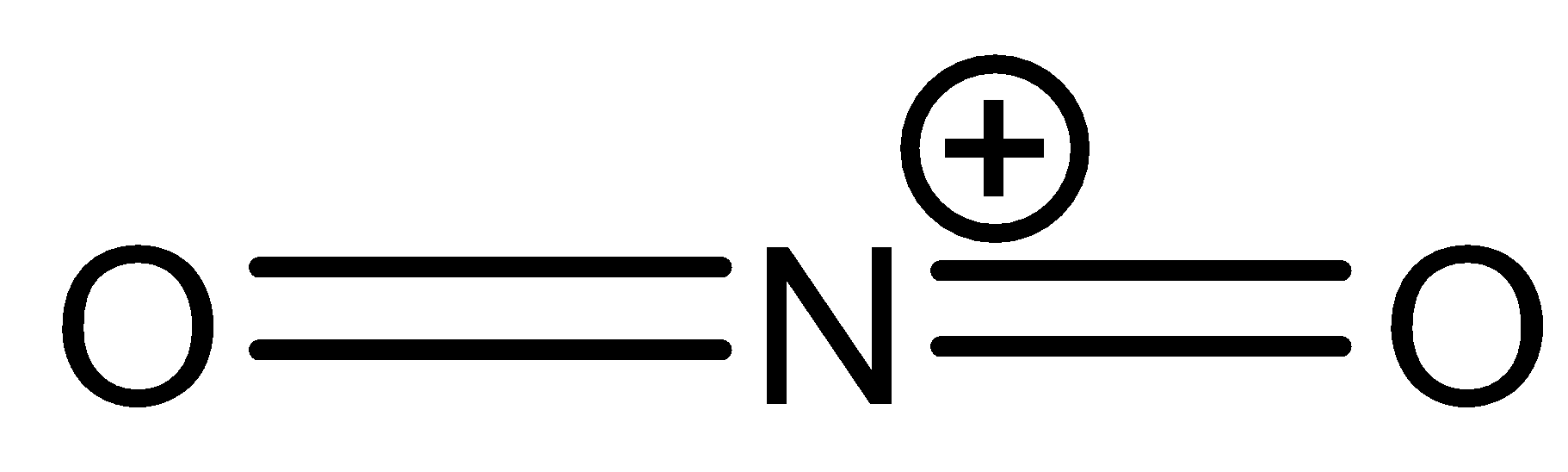
From the above structure, it is clear that the regions of electron density are two. Therefore the geometry of the molecule is linear according to VSEPR theory. For linear molecules, the central atom must undergo sp hybridization. The 2s and ${ 2p }_{ z }$ orbitals of nitrogen combine together in order to give two sp hybrid orbitals.
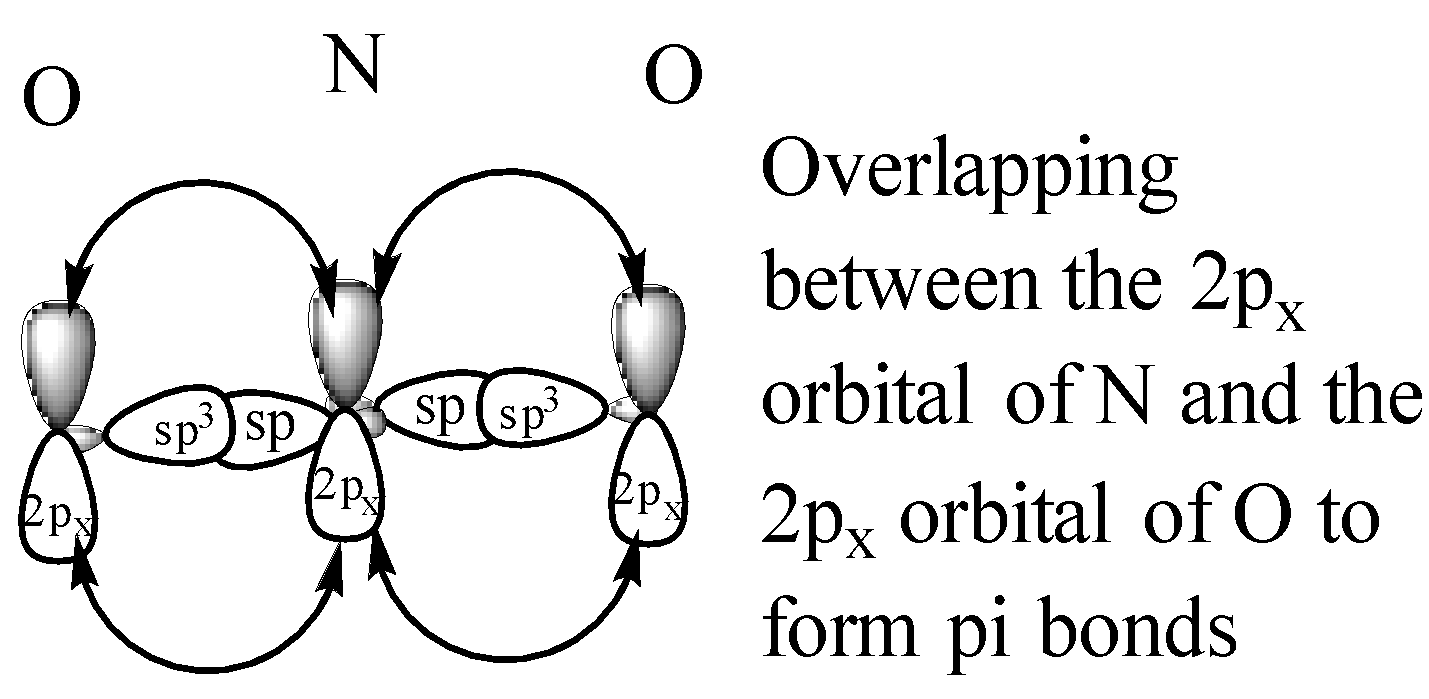
Now we will consider $N{ O }_{ 3 }^{ - }$
The Lewis structure is given below:
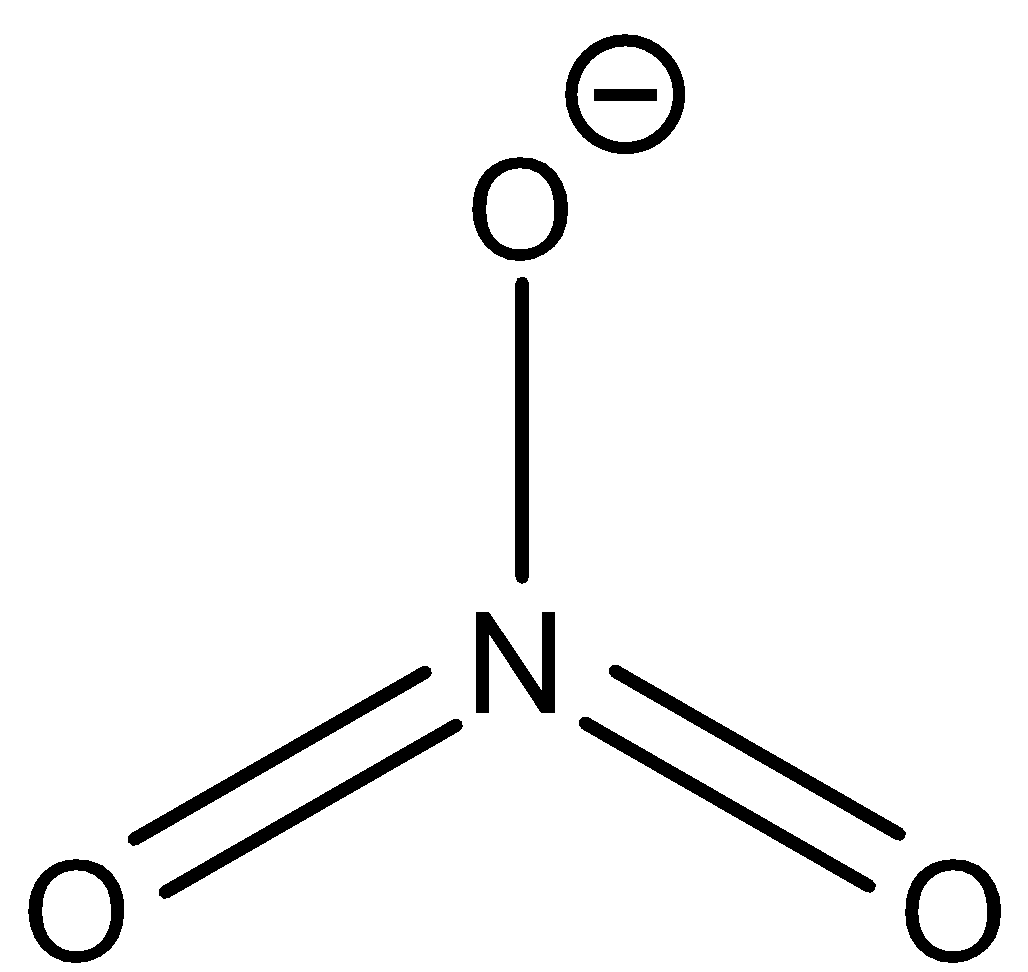
From the structure it is clear that the central atom has 3 regions of electron density, therefore its geometry should be a Trigonal planar according to VSEPR theory since the central atom has 2 bond pairs and 1 lone pair. The central atom undergoes $ { sp }^{ 2 }$ hybridization. The 2s and the two 2p orbitals combine together to give 3 $ { sp }^{ 2 }$ hybrid orbitals.
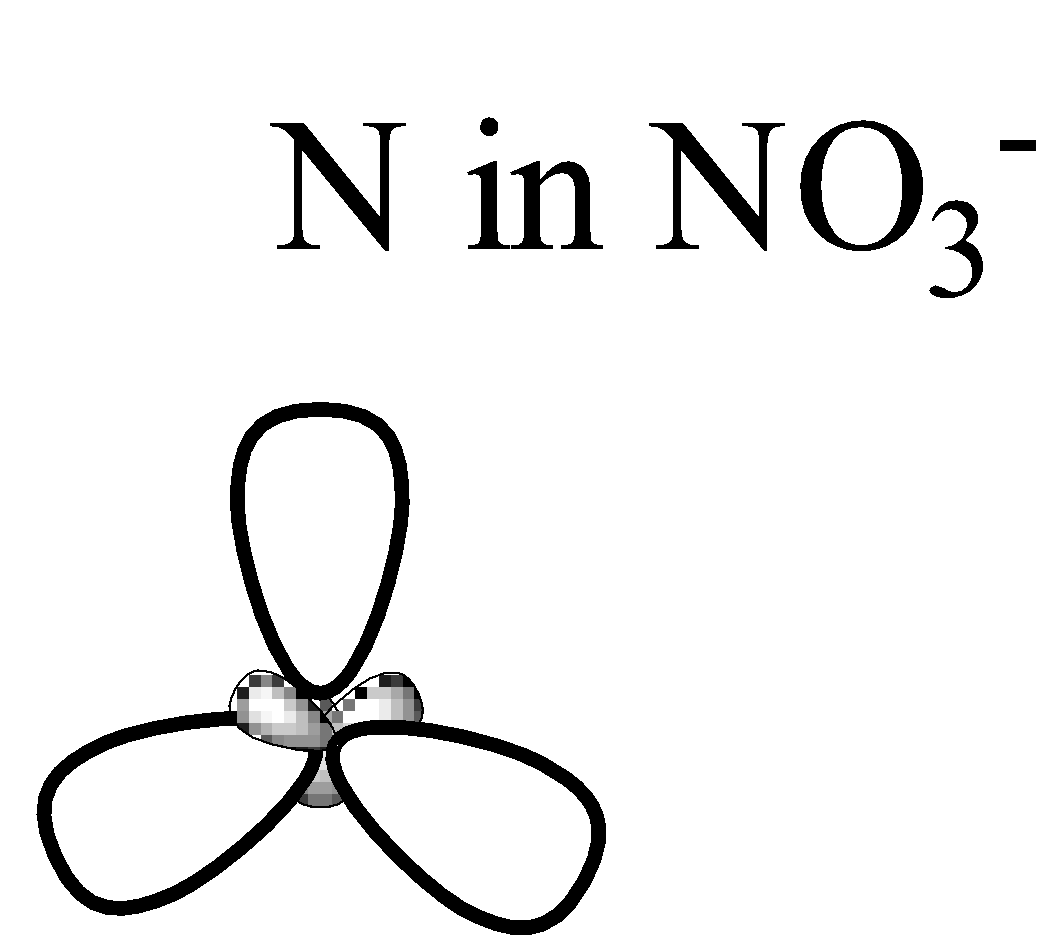
Now we will consider $N{ H }_{ 4 }^{ + }$
Its Lewis structure is given below:
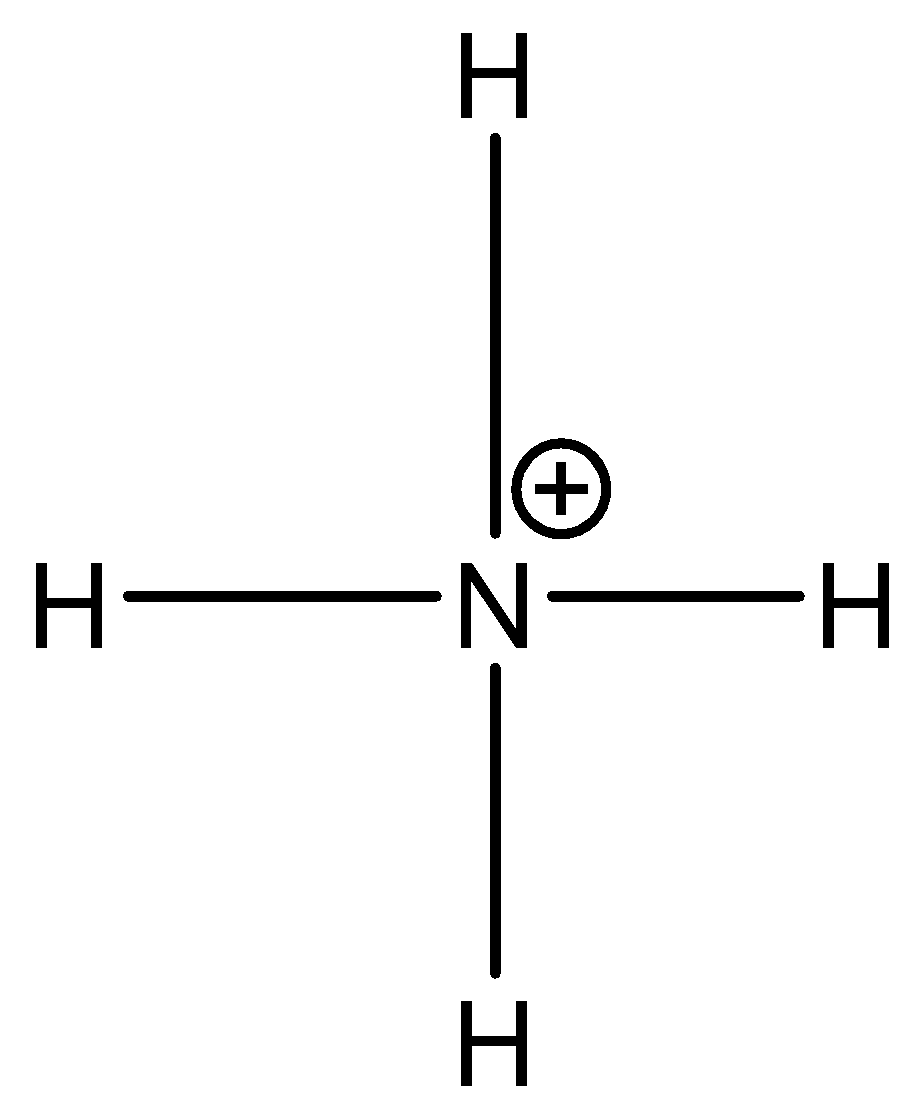
From the Lewis structure, we can see that the central atom has 4 regions of electron density. Therefore, its geometry will be Tetrahedral according to VESPR theory. The 2s and three 2p orbitals of nitrogen atom combine together in order to give four $ { sp }^{ 3 }$ hybrid orbitals.
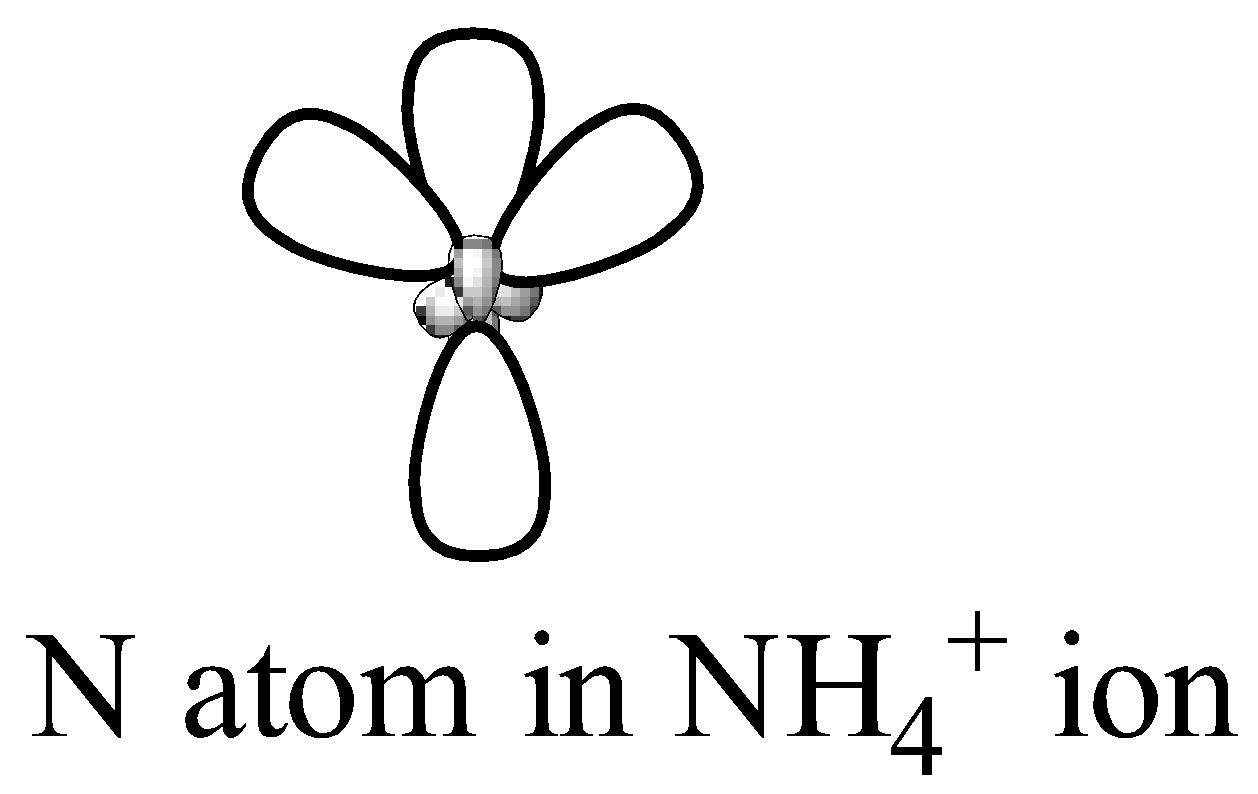
Therefore the option is (b) sp, $ { sp }^{ 2 }$ and $ { sp }^{ 3 }$ respectively.
Note: Hybridization and VSEPR theory can only be applied when the molecules contain small central atoms. It cannot explain the structure of transition metal compounds since it does not concern itself with the inactive lone pairs and the sizes of the substituent groups.
Complete step by step solution:
Hybridization is achieved through linear combination of atomic orbitals and the new orbitals are called hybrid orbitals. In order to determine the hybridization of an atom in a molecule we should follow the following steps:
-First you have to determine the Lewis structure of the molecule.
-Now assign the regions of electron density around the atom using VSEPR theory for predicting the shape of the molecule (single bonds, multiple bonds, radicals, and lone pairs each will count as one region).
-Now we can determine the set of hybridized orbitals corresponding to the geometry determined using VSEPR theory.
Let us start with $N{ O }_{ 2 }^{ + }$:
The Lewis structure of $N{ O }_{ 2 }^{ + }$ is:
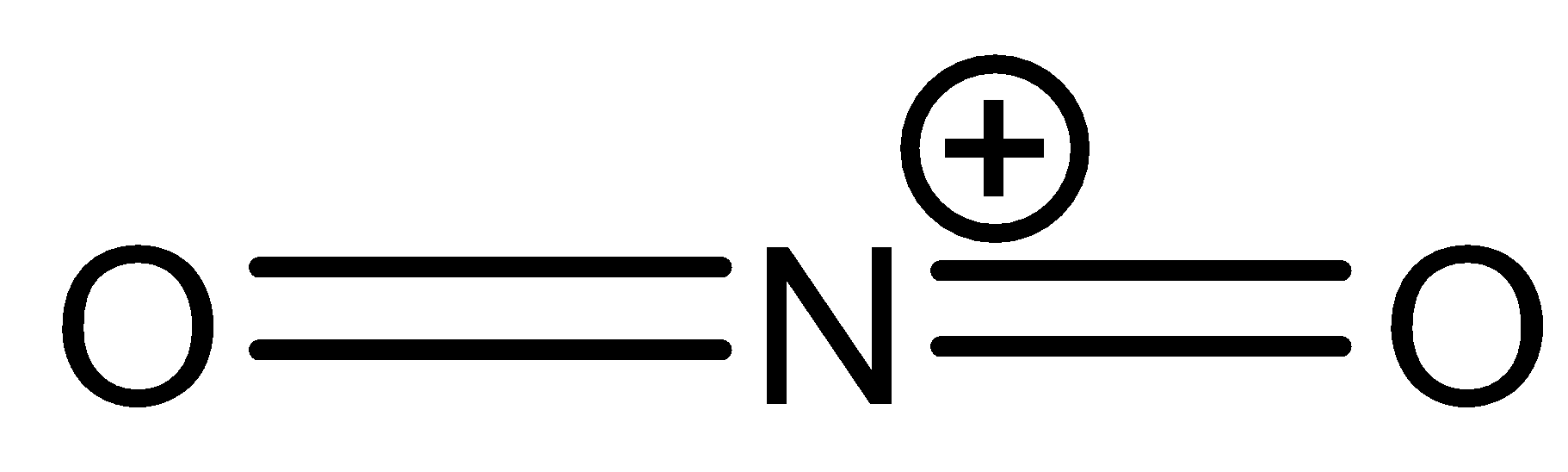
From the above structure, it is clear that the regions of electron density are two. Therefore the geometry of the molecule is linear according to VSEPR theory. For linear molecules, the central atom must undergo sp hybridization. The 2s and ${ 2p }_{ z }$ orbitals of nitrogen combine together in order to give two sp hybrid orbitals.

Now we will consider $N{ O }_{ 3 }^{ - }$
The Lewis structure is given below:

From the structure it is clear that the central atom has 3 regions of electron density, therefore its geometry should be a Trigonal planar according to VSEPR theory since the central atom has 2 bond pairs and 1 lone pair. The central atom undergoes $ { sp }^{ 2 }$ hybridization. The 2s and the two 2p orbitals combine together to give 3 $ { sp }^{ 2 }$ hybrid orbitals.

Now we will consider $N{ H }_{ 4 }^{ + }$
Its Lewis structure is given below:

From the Lewis structure, we can see that the central atom has 4 regions of electron density. Therefore, its geometry will be Tetrahedral according to VESPR theory. The 2s and three 2p orbitals of nitrogen atom combine together in order to give four $ { sp }^{ 3 }$ hybrid orbitals.

Therefore the option is (b) sp, $ { sp }^{ 2 }$ and $ { sp }^{ 3 }$ respectively.
Note: Hybridization and VSEPR theory can only be applied when the molecules contain small central atoms. It cannot explain the structure of transition metal compounds since it does not concern itself with the inactive lone pairs and the sizes of the substituent groups.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




