
The hybridisation of $BeC{l_2}$ in the solid state and above the $1200k$ respectively is :
$\left( 1 \right)s{p^3},s{p^3}$
$\left( 2 \right)s{p^3},s{p^2}$
$\left( 3 \right)s{p^2},s{p^2}$
$\left( 4 \right)s{p^3},sp$
Answer
558.6k+ views
Hint: Hybridization is a process in which the atomic orbitals merge to form a degenerated new orbitals. Here the $BeCl_2$, in which the atomic number of be is 4 and it has two electrons to share while forming a bond . The chlorine atoms will share each one electron .
Complete step by step answer:
$Cl - Be - Cl$
If we see becl2 then the above structure will come to our mind , but actually it is not true in the solid state of this molecule . In solid state two Chlorine atoms donate a pair of electrons to the beryllium in the ${45^ \circ }$ forming coordination bond. This will lead to form $4$bonds with the beryllium , two chlorine forms covalent bonds and two will form coordination bonds , which will give the hybridization$s{p^3}$. Basically we can say that in solid state the $BeC{l_2}$is present in the polymeric chain.
$$
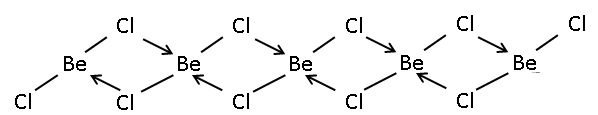
But if we look at the gaseous state , the $BeC{l_2}$is in equilibrium with both the linear $BeC{l_2}$ and the dimerized form ${\left( {BeC{l_2}} \right)_2}$ in dimerized form each beryllium is attached to three chlorine and thus giving it $s{p^2}$hybridization.
Here the question asked the hybridization above $1200k$ , in this case the dimerize form actually breaks leaving behind the linear form of becl2 . so in this case the hybridization will be $sp$ hybridization . so the answer is $\left( 4 \right)s{p^3},sp$
Additional information: Trick to find the hybridization of a molecule : $0.5\left( {V + M - C + A} \right)$
Where $S{p^3},S{p^3}$$V = $ number of Valence electrons
$M = $ Number of monovalent atoms
$C = $ Cation charge
$A = $ anion charge
Put all the value in the above formula and solve it . Then compare the answer with the following hybridization form .
$sp = 2$ $s{p^2} = 3$ $s{p^3} = 4$ $s{p^3}d = 5$ $s{p^3}{d^2} = 6$
Note: Try to remember the trick formula which will help you to find your answer easily . All the alkali , halogens and hydrogen are monovalent atoms . Remember the positive charge is to be subtracted and vice versa .
Complete step by step answer:
$Cl - Be - Cl$
If we see becl2 then the above structure will come to our mind , but actually it is not true in the solid state of this molecule . In solid state two Chlorine atoms donate a pair of electrons to the beryllium in the ${45^ \circ }$ forming coordination bond. This will lead to form $4$bonds with the beryllium , two chlorine forms covalent bonds and two will form coordination bonds , which will give the hybridization$s{p^3}$. Basically we can say that in solid state the $BeC{l_2}$is present in the polymeric chain.
$$

But if we look at the gaseous state , the $BeC{l_2}$is in equilibrium with both the linear $BeC{l_2}$ and the dimerized form ${\left( {BeC{l_2}} \right)_2}$ in dimerized form each beryllium is attached to three chlorine and thus giving it $s{p^2}$hybridization.
Here the question asked the hybridization above $1200k$ , in this case the dimerize form actually breaks leaving behind the linear form of becl2 . so in this case the hybridization will be $sp$ hybridization . so the answer is $\left( 4 \right)s{p^3},sp$
Additional information: Trick to find the hybridization of a molecule : $0.5\left( {V + M - C + A} \right)$
Where $S{p^3},S{p^3}$$V = $ number of Valence electrons
$M = $ Number of monovalent atoms
$C = $ Cation charge
$A = $ anion charge
Put all the value in the above formula and solve it . Then compare the answer with the following hybridization form .
$sp = 2$ $s{p^2} = 3$ $s{p^3} = 4$ $s{p^3}d = 5$ $s{p^3}{d^2} = 6$
Note: Try to remember the trick formula which will help you to find your answer easily . All the alkali , halogens and hydrogen are monovalent atoms . Remember the positive charge is to be subtracted and vice versa .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




