
The hybridisation of Ni in $Ni{{\left( CO \right)}_{4}}$ is:
(A) $s{{p}^{2}}$
(B) $ds{{p}^{2}}$
(C) $s{{p}^{3}}$
(D) $s{{p}^{3}}d$
Answer
588k+ views
Hint: To find out the hybridisation here approach using the valence bond theory. Start by writing down the electron configuration of the nickel atom. When the carbonyl group is attached, the nickel atoms will pair up and the carbonyl electrons will take up the remaining p-orbital. This will give us the required hybridisation.
Complete step by step solution: The compound given to us is $Ni{{\left( CO \right)}_{4}}$. Here, nickel is in a neutral state. We have to find its hybridisation. To find its hybridisation, firstly let us write down its electronic configuration.
Nickel is a 3d transition element. We can write its electronic configuration as- $\left[ Ar \right]3{{d}^{8}}4{{s}^{2}}$. We can see that in the given compound nickel is in a neutral state. By drawing the orbital diagram of the nickel we can find out the hybridisation.
Nickel has 3 electrons in d-orbital and 2 in s-orbital. Carbonyl is a strong field ligand and will form the inner orbital complex. Also, it forms weak spin complexes.
Now, when the carbonyl group is there, its electron will be in the 4s and 3p orbitals (as there are 4 carbonyl groups present). Thus, hybridisation of Ni will be $s{{p}^{3}}$ (as carbonyl electrons are taking up nickels empty s-orbital (one )and p-orbitals (three).
So, let us draw the orbital diagram for the 3d, 4s and 4p orbital for nickel ion and when it combines with carbonyl groups which are strong field ligands-
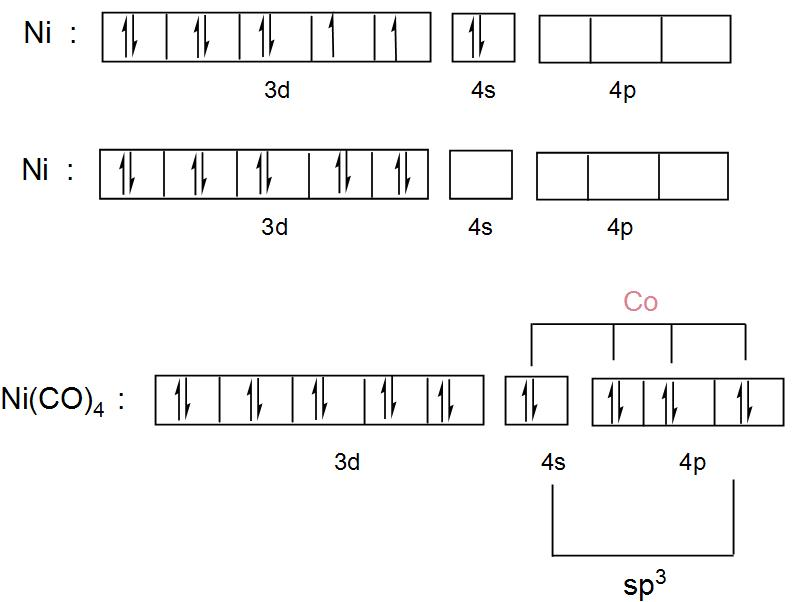
We can understand from the above discussion and the diagram that its hybridisation is $s{{p}^{3}}$and it forms an outer orbital complex as the carbonyl groups took the outer orbitals of the nickel atom.
Therefore, the correct answer is option [C] $s{{p}^{3}}$.
Note: Here we have used valence bond theory for estimation of hybridisation. There are certain limitations to this theory.
-The preferred geometry and colour of the complex cannot be explained.
-The distortion of the shape of a complex from regular geometry cannot be explained.
-The geometry of a complex cannot be predicted correctly using the magnetic moment data always.
-Classification that inner orbital complexes are covalent and outer orbital complexes are ionic is misleading.
Complete step by step solution: The compound given to us is $Ni{{\left( CO \right)}_{4}}$. Here, nickel is in a neutral state. We have to find its hybridisation. To find its hybridisation, firstly let us write down its electronic configuration.
Nickel is a 3d transition element. We can write its electronic configuration as- $\left[ Ar \right]3{{d}^{8}}4{{s}^{2}}$. We can see that in the given compound nickel is in a neutral state. By drawing the orbital diagram of the nickel we can find out the hybridisation.
Nickel has 3 electrons in d-orbital and 2 in s-orbital. Carbonyl is a strong field ligand and will form the inner orbital complex. Also, it forms weak spin complexes.
Now, when the carbonyl group is there, its electron will be in the 4s and 3p orbitals (as there are 4 carbonyl groups present). Thus, hybridisation of Ni will be $s{{p}^{3}}$ (as carbonyl electrons are taking up nickels empty s-orbital (one )and p-orbitals (three).
So, let us draw the orbital diagram for the 3d, 4s and 4p orbital for nickel ion and when it combines with carbonyl groups which are strong field ligands-

We can understand from the above discussion and the diagram that its hybridisation is $s{{p}^{3}}$and it forms an outer orbital complex as the carbonyl groups took the outer orbitals of the nickel atom.
Therefore, the correct answer is option [C] $s{{p}^{3}}$.
Note: Here we have used valence bond theory for estimation of hybridisation. There are certain limitations to this theory.
-The preferred geometry and colour of the complex cannot be explained.
-The distortion of the shape of a complex from regular geometry cannot be explained.
-The geometry of a complex cannot be predicted correctly using the magnetic moment data always.
-Classification that inner orbital complexes are covalent and outer orbital complexes are ionic is misleading.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




