
The hybridization, lone pairs of electron and shape of $I_3^ - $ is:
a.) It has $s{p^3}d$ hybridization
b.) It has trigonal bi-pyramidal shape
c.) It is linear
d.) It has 3 lone pair of electrons
Answer
599.1k+ views
Hint: In order to deal with this question we have to keep in mind that $I_3^ - $ is a linear anion. It is formed by the bonding of ${I_2}$ with ${I^ - }$ ion. Here ${I^ - }$ ion will be the donor and ${I_2}$ molecule will be the acceptor where electrons are usually accommodated in the empty d orbitals. Use this concept to reach the answer.
Complete answer:
We can determine the hybridization of $I_3^ - $ by knowing the number of valence electrons and lone pairs and calculating their sum. In this case, if we consider the lone pairs there are 3 such pairs while the number of atoms donating valence electrons is 2. If we add these values together we get 5 which can be interpreted as sp3d hybridization
• $I_3^ - $ is formed by the bonding of ${I_2}$ with I ion.
• During the combination of Iodine atoms, the central atom gains a negative charge whose value will be 1.
• ${I^ - }$ ion is the donor and ${I_2}$ molecule is the acceptor. Electrons are mostly accommodated in the empty d orbitals.
Structure :
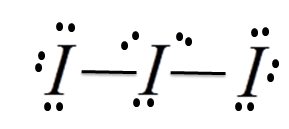
$I_3^ - $ molecular geometry is linear. While there are three Iodine atoms, one of the atoms has a negative charge which further gives 3 lone pairs of electrons and 2 bond pairs. Its steric number will be 5. The three lone pairs will repel each other and take up equatorial positions. The remaining two Iodine atoms are at 180o from each other.
Hence the options are A, C, D
Note: In order to solve these types of questions, you need to remember the structure and hybridisation of these types of molecules. You also need to have a good concept of hybridization theory and how to calculate the hybridization of a molecule. One of the simple methods is to draw the structure of the molecule and then count the number of sigma bonds, then you can tell the hybridization of the molecule but you have to remember the structure of the molecule.
Complete answer:
We can determine the hybridization of $I_3^ - $ by knowing the number of valence electrons and lone pairs and calculating their sum. In this case, if we consider the lone pairs there are 3 such pairs while the number of atoms donating valence electrons is 2. If we add these values together we get 5 which can be interpreted as sp3d hybridization
• $I_3^ - $ is formed by the bonding of ${I_2}$ with I ion.
• During the combination of Iodine atoms, the central atom gains a negative charge whose value will be 1.
• ${I^ - }$ ion is the donor and ${I_2}$ molecule is the acceptor. Electrons are mostly accommodated in the empty d orbitals.
Structure :
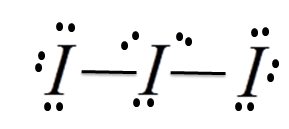
$I_3^ - $ molecular geometry is linear. While there are three Iodine atoms, one of the atoms has a negative charge which further gives 3 lone pairs of electrons and 2 bond pairs. Its steric number will be 5. The three lone pairs will repel each other and take up equatorial positions. The remaining two Iodine atoms are at 180o from each other.
Hence the options are A, C, D
Note: In order to solve these types of questions, you need to remember the structure and hybridisation of these types of molecules. You also need to have a good concept of hybridization theory and how to calculate the hybridization of a molecule. One of the simple methods is to draw the structure of the molecule and then count the number of sigma bonds, then you can tell the hybridization of the molecule but you have to remember the structure of the molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




