
The hybridization of atomic orbitals of N in $NO_{2}^{+}$, $NO_{3}^{-}$ and $NH_{4}^{+}$ are respectively.
(A) \[sp\], \[s{{p}^{2}}\], \[s{{p}^{3}}\]
(B) \[sp\],\[s{{p}^{3}}\], \[s{{p}^{2}}\]
(C) \[s{{p}^{2}}\], \[sp\], \[s{{p}^{3}}\]
(D) \[s{{p}^{2}}\], \[s{{p}^{3}}\], \[sp\]
Answer
596.1k+ views
Hint: Hybridization of a molecule is determined using the postulates of the Valence band theory and it is basically the phenomenon of mixing of orbitals.
Complete step by step solution:
Hybridization (mixing of orbitals) when two or more than two types of orbitals having less energy difference are combined and form the same number of new orbitals having similar shape and energy but different orientation.
Formula of hybridization; -
$H=\dfrac{(V+M-C+A)}{2}$
Here,
H= hybridization
V = number of valence shell electron of central atom
M= number of monovalent atom surrounding the atom
C= charge on cation
A= charge on anion
-First of all, we have to find out which is the central Atom here so the nitrogen is the central Atom here.
-Therefore, the electronic configuration of nitrogen atom is 1\[{{s}^{2}}\] 2\[{{s}^{2}}\] 2\[{{p}^{3}}\].
-According to the electronic configuration the first (inner) shell will have two electrons and the outermost shell will have two electrons and the outermost shell will have 5 electrons.
-Number of outermost shell electrons is the number of valence shell electrons of Atom.
So, now we are solving for $NO_{2}^{+}$;
By formula of hybridization
$H=\dfrac{(V+M-C+A)}{2}$
where,
V= 5; C= 1
M= 0; A= 0
Put these values in formula
H =$\dfrac{\left( 5\text{ }+\text{ }0\text{ }-1\text{ }+\text{ }0\text{ } \right)}{2}$
H= $\dfrac{4}{2}$= 2
So, the hybridization = \[sp\]

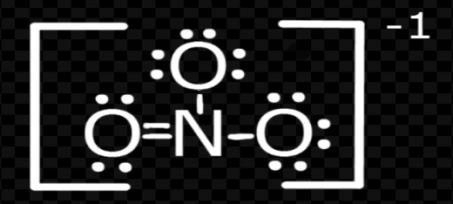
Now solving for $NO_{3}^{-}$;
Again, by formula
V = 5; M= 0
C= 0; A = 1
Put these values in formula
$H=\dfrac{(V+M-C+A)}{2}$
H= \[\dfrac{\left( 5\text{ }+\text{ }0\text{ }-\text{ }0\text{ }+\text{ }1 \right)}{2}\]
H= $\dfrac{6}{2}$ = 3
So, the hybridization = \[s{{p}^{2}}\]
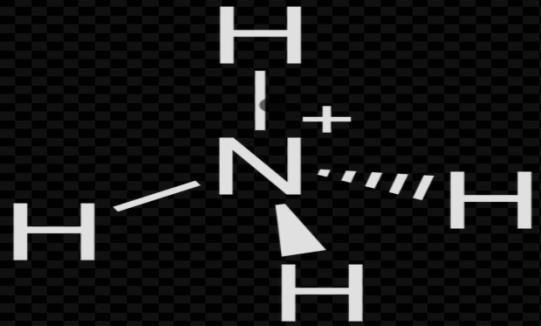
Now solving for $NH_{4}^{+}$;
Again, by formula
$H=\dfrac{(V+M-C+A)}{2}$
V= 5; M = 4
C = 1; A = 0
Put these values in formula
H= $\dfrac{\left( 5\text{ }+\text{ }4\text{ }-\text{ }1\text{ }+\text{ }0 \right)}{2}$
H=$\dfrac{8}{2}$= 4
So, the hybridization is =\[s{{p}^{3}}\]
So, by solving these the hybridization of atomic orbitals of N in $NO_{2}^{+}$, $NO_{3}^{-}$ and$NH_{4}^{+}$ are respectively \[sp\], \[s{{p}^{2}}\], \[s{{p}^{3}}\].
Therefore, the correct option is option (A) \[sp\], \[s{{p}^{2}}\], \[s{{p}^{3}}\].
Note:
-Check properly the number of valence shell electrons of the central Atom.
-Check properly the number of monovalent atoms.
-Check properly the charge of cation and anions
Complete step by step solution:
Hybridization (mixing of orbitals) when two or more than two types of orbitals having less energy difference are combined and form the same number of new orbitals having similar shape and energy but different orientation.
Formula of hybridization; -
$H=\dfrac{(V+M-C+A)}{2}$
Here,
H= hybridization
V = number of valence shell electron of central atom
M= number of monovalent atom surrounding the atom
C= charge on cation
A= charge on anion
-First of all, we have to find out which is the central Atom here so the nitrogen is the central Atom here.
-Therefore, the electronic configuration of nitrogen atom is 1\[{{s}^{2}}\] 2\[{{s}^{2}}\] 2\[{{p}^{3}}\].
-According to the electronic configuration the first (inner) shell will have two electrons and the outermost shell will have two electrons and the outermost shell will have 5 electrons.
-Number of outermost shell electrons is the number of valence shell electrons of Atom.
So, now we are solving for $NO_{2}^{+}$;
By formula of hybridization
$H=\dfrac{(V+M-C+A)}{2}$
where,
V= 5; C= 1
M= 0; A= 0
Put these values in formula
H =$\dfrac{\left( 5\text{ }+\text{ }0\text{ }-1\text{ }+\text{ }0\text{ } \right)}{2}$
H= $\dfrac{4}{2}$= 2
So, the hybridization = \[sp\]

Shape = linear
Now solving for $NO_{3}^{-}$;
Again, by formula
V = 5; M= 0
C= 0; A = 1
Put these values in formula
$H=\dfrac{(V+M-C+A)}{2}$
H= \[\dfrac{\left( 5\text{ }+\text{ }0\text{ }-\text{ }0\text{ }+\text{ }1 \right)}{2}\]
H= $\dfrac{6}{2}$ = 3
So, the hybridization = \[s{{p}^{2}}\]

Shape = trigonal planar
Now solving for $NH_{4}^{+}$;
Again, by formula
$H=\dfrac{(V+M-C+A)}{2}$
V= 5; M = 4
C = 1; A = 0
Put these values in formula
H= $\dfrac{\left( 5\text{ }+\text{ }4\text{ }-\text{ }1\text{ }+\text{ }0 \right)}{2}$
H=$\dfrac{8}{2}$= 4
So, the hybridization is =\[s{{p}^{3}}\]

Shape = tetrahedral
So, by solving these the hybridization of atomic orbitals of N in $NO_{2}^{+}$, $NO_{3}^{-}$ and$NH_{4}^{+}$ are respectively \[sp\], \[s{{p}^{2}}\], \[s{{p}^{3}}\].
Therefore, the correct option is option (A) \[sp\], \[s{{p}^{2}}\], \[s{{p}^{3}}\].
Note:
-Check properly the number of valence shell electrons of the central Atom.
-Check properly the number of monovalent atoms.
-Check properly the charge of cation and anions
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




