
The hybridization of \[N\] atom in \[NO_3^ - \] ,\[NO_2^ - \] and \[NH_4^ - \] respectively is:
A.\[s{p^2},s{p^3},sp\]
B.\[sp,s{p^3},s{p^2}\]
C.\[sp,s{p^2},s{p^3}\]
D.\[s{p^2},sp,s{p^3}\]
Answer
559.2k+ views
Hint: We need to know that the orbital hybridization in chemistry is the concept of combining atomic orbitals into new hybrid orbitals suitable for pairing electrons in the theory of valence bonds to form chemical bonds.
As we know that when an atom binds using electrons from both the orbitals s and p, hybridization happens, producing an imbalance between the electrons' energy levels. The s and p orbitals involved are combined to form hybrid orbitals to equalize these energy levels.
Complete step by step answer:
The N atom hybridization in \[NO_3^ - \], \[NO_2^ - \] and \[NH_4^ - \] is \[s{p^2},sp,s{p^3}\], respectively.
In \[NO_3^ - \] there are 3 bonding domains in the central N atom (one single bond and two double bonds) and zero lone electron pairs. We can draw the structure of this compound as,
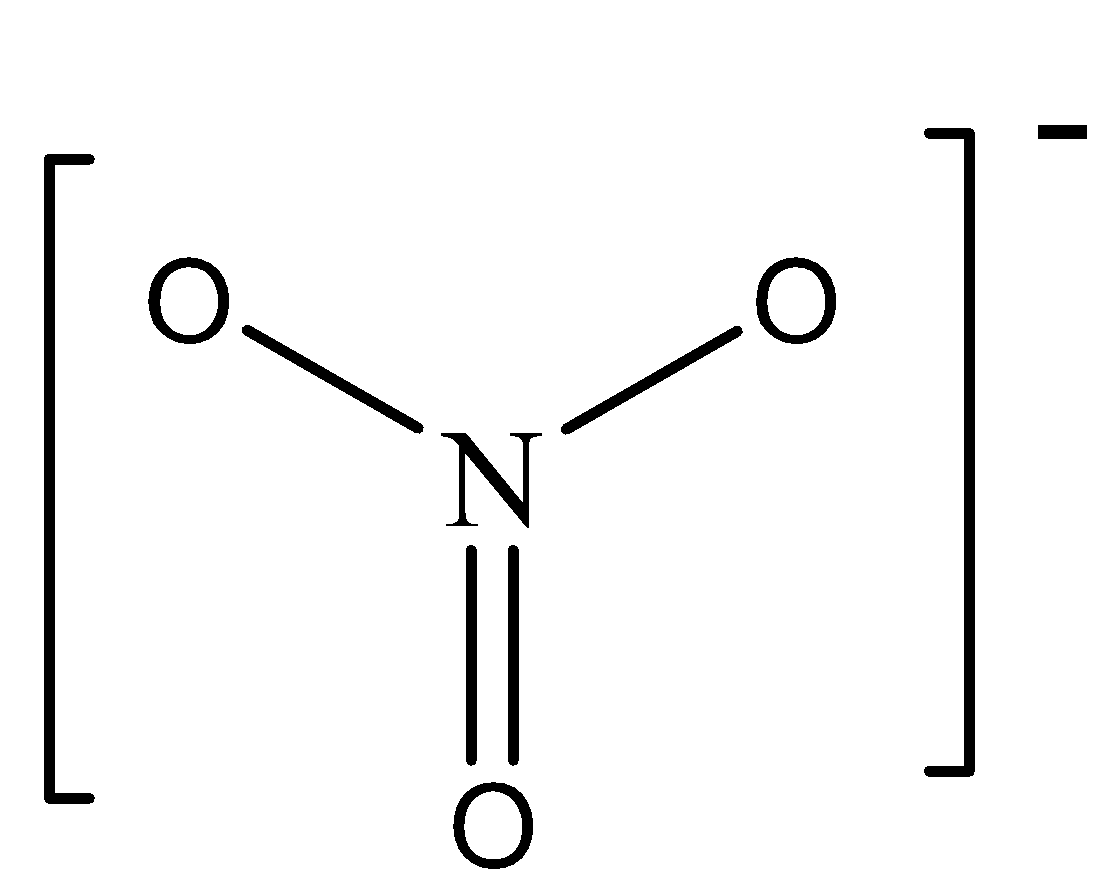
In \[NO_2^ - \], there are 2 bonding domains in the central N atom (one single bond and one double bond) and zero lone electron pairs. We can draw the structure of this compound as,
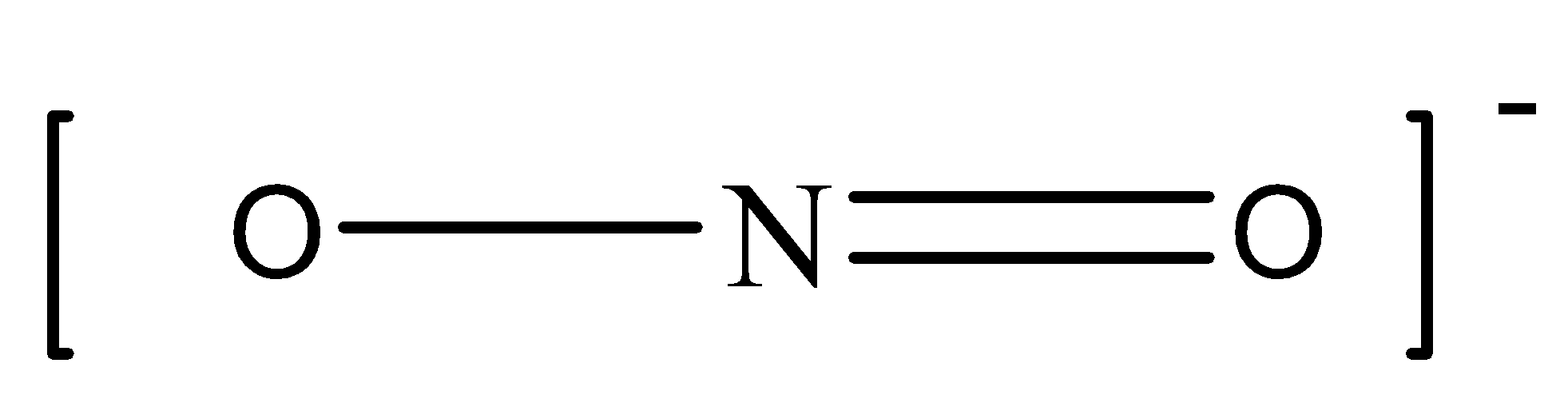
In \[NH_4^ - \] , there are 4 bonding domains (4 single bonds and zero lone electron pairs in the central N atom. We can draw the structure of this compound as,

Option D is the correct option as the given hybridization is correct for the given atoms respectively.
Note:
With the exception of the first three noble gases, helium, neon, and argon, and some of the very short-lived elements after bismuth, nitrogen binds to almost all the elements in the periodic table, producing an enormous range of binary compounds with varying properties and applications. Many binary compounds are known: these are usually labelled n, with the exception of nitrogen hydrides, oxides, and fluorides.
We have to know that the orbital hybridization (or hybridization) in chemistry is the process of combining atomic orbitals into new hybrid orbitals (with different energies, sizes, etc., than atomic orbitals) suitable for pairing electrons in valence bond theory to form chemical bonds.
As we know that when an atom binds using electrons from both the orbitals s and p, hybridization happens, producing an imbalance between the electrons' energy levels. The s and p orbitals involved are combined to form hybrid orbitals to equalize these energy levels.
Complete step by step answer:
The N atom hybridization in \[NO_3^ - \], \[NO_2^ - \] and \[NH_4^ - \] is \[s{p^2},sp,s{p^3}\], respectively.
In \[NO_3^ - \] there are 3 bonding domains in the central N atom (one single bond and two double bonds) and zero lone electron pairs. We can draw the structure of this compound as,

In \[NO_2^ - \], there are 2 bonding domains in the central N atom (one single bond and one double bond) and zero lone electron pairs. We can draw the structure of this compound as,
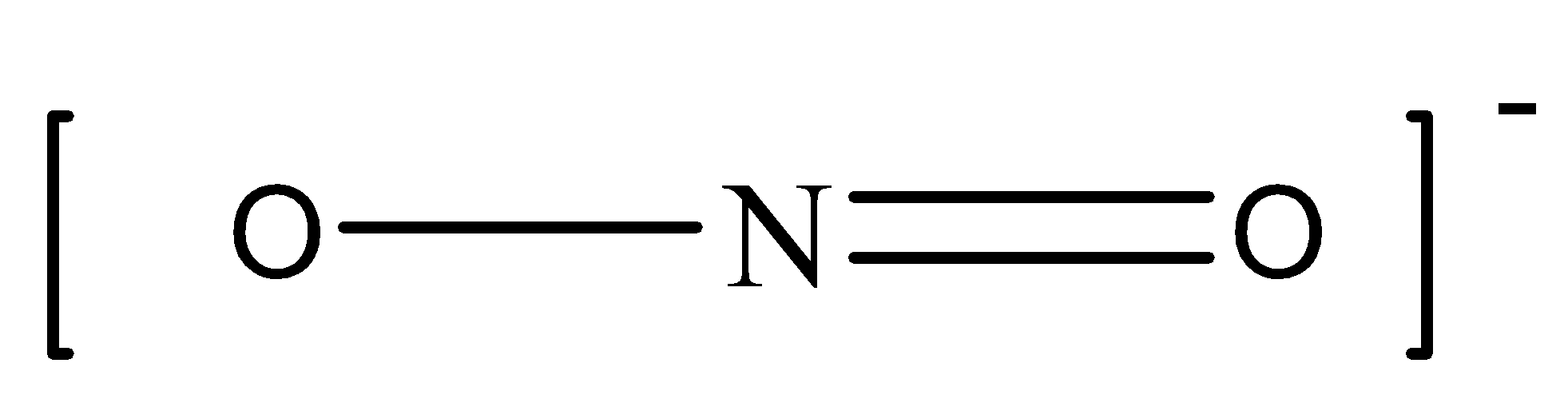
In \[NH_4^ - \] , there are 4 bonding domains (4 single bonds and zero lone electron pairs in the central N atom. We can draw the structure of this compound as,

Option D is the correct option as the given hybridization is correct for the given atoms respectively.
Note:
With the exception of the first three noble gases, helium, neon, and argon, and some of the very short-lived elements after bismuth, nitrogen binds to almost all the elements in the periodic table, producing an enormous range of binary compounds with varying properties and applications. Many binary compounds are known: these are usually labelled n, with the exception of nitrogen hydrides, oxides, and fluorides.
We have to know that the orbital hybridization (or hybridization) in chemistry is the process of combining atomic orbitals into new hybrid orbitals (with different energies, sizes, etc., than atomic orbitals) suitable for pairing electrons in valence bond theory to form chemical bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




