
The hybridization of $NH_4^ + $ ion is:
A. $sp$
B. $s{p^2}$
C. $s{p^3}$
D. $s{p^3}d$
Answer
577.5k+ views
Hint: In chemistry, the orbital hybridization is the concept of mixing atomic orbitals of different energies into new hybrid orbitals of same energy levels but with different energies than the previous individual atomic ones, different in shapes, etc., suitable for the pairing of electrons to form chemical bonds in valence bond theory.
Complete step by step answer:
Orbitals are a model representation of the behavior of electrons within molecules. The orbitals are space inside the atom where there is a maximum probability of finding an electron. In the case of simple hybridization, this approximation is based on atomic orbitals, similar to those obtained for the hydrogen atom, the only neutral atom for which the Schrödinger equation can be solved exactly. In heavier atoms, such as carbon, nitrogen, and oxygen, the atomic orbitals used are the \[2s\] and \[2p\] orbitals, similar to excited state orbitals for hydrogen. Hybrid orbitals are assumed to be mixtures of atomic orbitals, superimposed on each other in various proportions.
In the case of an ammonium ion ($NH_4^ + $), the hybridization can be determined by the general formula:
Number of hybrid orbitals = $\dfrac{1}{2}(N + B - C)$
Where, \[N\] is the number of valence electrons in the central atom, $B$ is the number of atoms surrounding it and $C$ is the total charge on the atom.
As per the given ion, $N = 5$
$B = 4$
$C = 1$
Thus, substituting the values and solving, we have:
Number of hybrid orbitals = $\dfrac{1}{2}(5 + 4 - 1) = 4$
This means that the total number of atomic orbitals that are needed to constitute 4 hybrid orbitals is = 4.
Hence, the hybridization of ammonium ion ($NH_4^ + $) = $s{p^3}$
So, the correct answer is “Option A”.
Note:
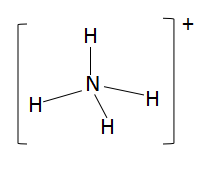
As ammonium ion is $s{p^3}$hybridized, this means that it is tetrahedral in structure and all the bonds are substituted to each other at equal angles. The possible structure of the ammonium ion is as follows:
Complete step by step answer:
Orbitals are a model representation of the behavior of electrons within molecules. The orbitals are space inside the atom where there is a maximum probability of finding an electron. In the case of simple hybridization, this approximation is based on atomic orbitals, similar to those obtained for the hydrogen atom, the only neutral atom for which the Schrödinger equation can be solved exactly. In heavier atoms, such as carbon, nitrogen, and oxygen, the atomic orbitals used are the \[2s\] and \[2p\] orbitals, similar to excited state orbitals for hydrogen. Hybrid orbitals are assumed to be mixtures of atomic orbitals, superimposed on each other in various proportions.
In the case of an ammonium ion ($NH_4^ + $), the hybridization can be determined by the general formula:
Number of hybrid orbitals = $\dfrac{1}{2}(N + B - C)$
Where, \[N\] is the number of valence electrons in the central atom, $B$ is the number of atoms surrounding it and $C$ is the total charge on the atom.
As per the given ion, $N = 5$
$B = 4$
$C = 1$
Thus, substituting the values and solving, we have:
Number of hybrid orbitals = $\dfrac{1}{2}(5 + 4 - 1) = 4$
This means that the total number of atomic orbitals that are needed to constitute 4 hybrid orbitals is = 4.
Hence, the hybridization of ammonium ion ($NH_4^ + $) = $s{p^3}$
So, the correct answer is “Option A”.
Note:
As ammonium ion is $s{p^3}$hybridized, this means that it is tetrahedral in structure and all the bonds are substituted to each other at equal angles. The possible structure of the ammonium ion is as follows:

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




