
The hybridization of ${[NiC{l_4}]^{2 - }}$ and ${[Ni{(CN)_4}]^{2 - }}$ considering hybridization of the metal ion are respectively.
A.$s{p^3},ds{p^2}$
B.$ds{p^2},s{p^3}$
C.Both $s{p^3}$
D.Both $ds{p^2}$
Answer
584.4k+ views
Hint: Hybridization is defined as the concept of intermixing of the orbitals of an atom having nearly the same energy to give exactly equivalent orbitals with the same energy, identical shapes, and symmetrical orientation in space.
Complete step by step answer:
In the case of the coordination compound, we will find the hybridization with the help of valence bond theory. In the case of ${[NiC{l_4}]^{2 - }}$, first we find the oxidation state of the central metal atom i.e. $Ni$. Let us consider the oxidation state of $Ni$ is x. Since chlorine is halogen, hence the oxidation state of chlorine is -1. There for the oxidation state of $Ni$ is calculated as below:
$x + 4 \times ( - 1) = - 2$
$ \Rightarrow x - 4 = - 2$
$ \Rightarrow x = - 2 + 4 = 2$
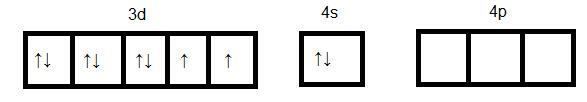
Hence the oxidation state of $Ni$is +2 and since the atomic number $Ni$ is 28. Its outer electronic configuration is $3{d^8}4{s^2}$ and it is represented as below:
$Ni$ in ground state:
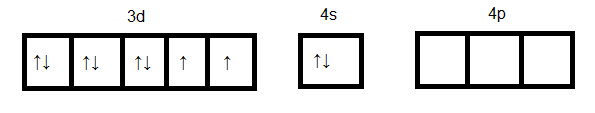
$Ni$ in +2 oxidation state:
Formation of ${[NiC{l_4}]^{2 - }}$
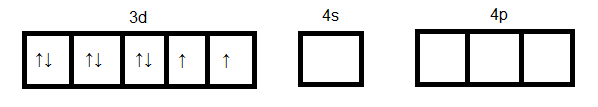
As we see in the above case d orbital cannot participate in hybridization. Four $s{p^3}$ orbitals can be formed by mixing one $4s$ and three $4p$ orbitals. So four hybrid orbitals accommodate four pairs of electrons from four chloride ions. Hence the hybridization will be $s{p^3}$ and geometry will be tetrahedral
In the case of ${[Ni{(CN)_4}]^{2 - }}$, we will again find the oxidation state of nickel. Suppose the oxidation state of nickel is x and the charge present on cyanide ion is a negative one and the overall charge present is negative two. Hence, the value of x can be calculated as:
$x + 4( - 1) = - 2$
$ \Rightarrow x = - 2 + 4 = 2$
Hence the oxidation state of nickel is +2 and its state is represented below
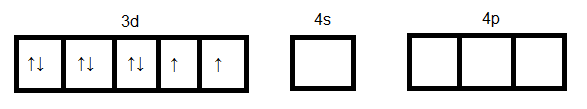
$Ni$ in ground state:
$Ni$ in +2 oxidation state:
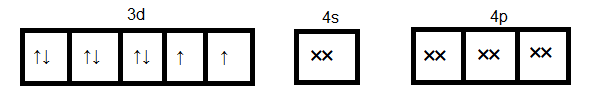
Since cyanide is a strong ligand hence pairing of electrons occurs. Formation of ${[Ni{(CN)_4}]^{2 - }}$
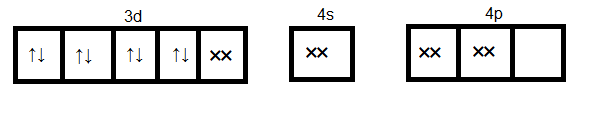
Here $ds{p^2}$ orbitals accommodate four pairs of the electron from four cyanide ions. Hence the hybridization is $ds{p^2}$ and geometry will be a square planner
Hence the correct answer is option is A.
Note:
Here ${[NiC{l_4}]^{2 - }}$ is paramagnetic due to the presence of an unpaired electron whereas in case of ${[Ni{(CN)_4}]^{2 - }}$ due to the presence of a paired electron it is diamagnetic.
Complete step by step answer:
In the case of the coordination compound, we will find the hybridization with the help of valence bond theory. In the case of ${[NiC{l_4}]^{2 - }}$, first we find the oxidation state of the central metal atom i.e. $Ni$. Let us consider the oxidation state of $Ni$ is x. Since chlorine is halogen, hence the oxidation state of chlorine is -1. There for the oxidation state of $Ni$ is calculated as below:
$x + 4 \times ( - 1) = - 2$
$ \Rightarrow x - 4 = - 2$
$ \Rightarrow x = - 2 + 4 = 2$
Hence the oxidation state of $Ni$is +2 and since the atomic number $Ni$ is 28. Its outer electronic configuration is $3{d^8}4{s^2}$ and it is represented as below:
$Ni$ in ground state:

$Ni$ in +2 oxidation state:

Formation of ${[NiC{l_4}]^{2 - }}$

As we see in the above case d orbital cannot participate in hybridization. Four $s{p^3}$ orbitals can be formed by mixing one $4s$ and three $4p$ orbitals. So four hybrid orbitals accommodate four pairs of electrons from four chloride ions. Hence the hybridization will be $s{p^3}$ and geometry will be tetrahedral
In the case of ${[Ni{(CN)_4}]^{2 - }}$, we will again find the oxidation state of nickel. Suppose the oxidation state of nickel is x and the charge present on cyanide ion is a negative one and the overall charge present is negative two. Hence, the value of x can be calculated as:
$x + 4( - 1) = - 2$
$ \Rightarrow x = - 2 + 4 = 2$
Hence the oxidation state of nickel is +2 and its state is represented below
$Ni$ in ground state:

$Ni$ in +2 oxidation state:

Since cyanide is a strong ligand hence pairing of electrons occurs. Formation of ${[Ni{(CN)_4}]^{2 - }}$

Here $ds{p^2}$ orbitals accommodate four pairs of the electron from four cyanide ions. Hence the hybridization is $ds{p^2}$ and geometry will be a square planner
Hence the correct answer is option is A.
Note:
Here ${[NiC{l_4}]^{2 - }}$ is paramagnetic due to the presence of an unpaired electron whereas in case of ${[Ni{(CN)_4}]^{2 - }}$ due to the presence of a paired electron it is diamagnetic.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




