
The hybridization state of the central atom in $\text{HgC}{{\text{l}}_{2}}$ is –
(1) $\text{sp}$
(2) $\text{s}{{\text{p}}^{2}}$
(3) $\text{s}{{\text{p}}^{3}}$
Answer
531.9k+ views
Hint: The structure of $\text{HgC}{{\text{l}}_{\text{2}}}$ is linear and the hybridization state of the central atom can be predicted by using VSEPR theory. This theory defines the hybridization and geometry of a compound by determining the number of electrons involved in bonding.
Complete answer:
To find out the hybridization of the central atom, we need to calculate the steric number. The steric number is the number of atoms bonded to a central atom of a molecule plus the no. of lone pairs attached to it. It is used alongside VSEPR theory to determine the molecular geometry and thus the hybridization of the central atom.
The atomic number of Hg is 80 and thus its electronic configuration is –
\[\text{Hg}-\left[ \text{Xe} \right]4{{\text{f}}^{14}}5{{\text{d}}^{10}}6{{\text{s}}^{2}}\]
Here, the outermost orbital is 6s-orbital. So, it has 2 valence electrons. Since it is bonded to two chlorine atoms, therefore the two electrons will be shared with these two chlorine atoms and it will form $\text{HgC}{{\text{l}}_{2}}$. Thus, it has no lone pairs of electrons. Therefore, the steric number of $\text{HgC}{{\text{l}}_{\text{2}}}$ comes out to be 2.
\[\begin{align}
& \text{Steric number}=\text{No}\text{. of atoms bonded to central atom}+\text{No}\text{. of lone pairs} \\
& \text{Steric number}\left( \text{HgC}{{\text{l}}_{2}} \right)=2+0=2 \\
\end{align}\]
This suggests that the molecule possesses linear geometry in which the hybridization of the central atom is sp-hybridized. The Lewis structure of $\text{HgC}{{\text{l}}_{2}}$ is drawn below.
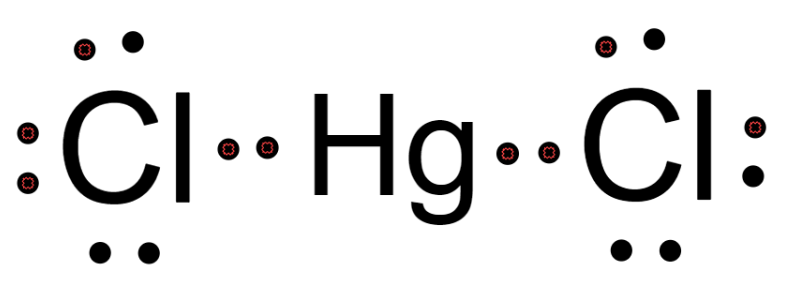
Hence, the correct answer is (1) sp.
Note:
The steric number defines the shape and geometry of the molecule by assigning the position of atoms in such a way that the repulsion between electron pairs will be minimal. For example, a steric number of 2 provides a linear shape in which the bonding electrons are 180 degrees apart.
Complete answer:
To find out the hybridization of the central atom, we need to calculate the steric number. The steric number is the number of atoms bonded to a central atom of a molecule plus the no. of lone pairs attached to it. It is used alongside VSEPR theory to determine the molecular geometry and thus the hybridization of the central atom.
The atomic number of Hg is 80 and thus its electronic configuration is –
\[\text{Hg}-\left[ \text{Xe} \right]4{{\text{f}}^{14}}5{{\text{d}}^{10}}6{{\text{s}}^{2}}\]
Here, the outermost orbital is 6s-orbital. So, it has 2 valence electrons. Since it is bonded to two chlorine atoms, therefore the two electrons will be shared with these two chlorine atoms and it will form $\text{HgC}{{\text{l}}_{2}}$. Thus, it has no lone pairs of electrons. Therefore, the steric number of $\text{HgC}{{\text{l}}_{\text{2}}}$ comes out to be 2.
\[\begin{align}
& \text{Steric number}=\text{No}\text{. of atoms bonded to central atom}+\text{No}\text{. of lone pairs} \\
& \text{Steric number}\left( \text{HgC}{{\text{l}}_{2}} \right)=2+0=2 \\
\end{align}\]
This suggests that the molecule possesses linear geometry in which the hybridization of the central atom is sp-hybridized. The Lewis structure of $\text{HgC}{{\text{l}}_{2}}$ is drawn below.
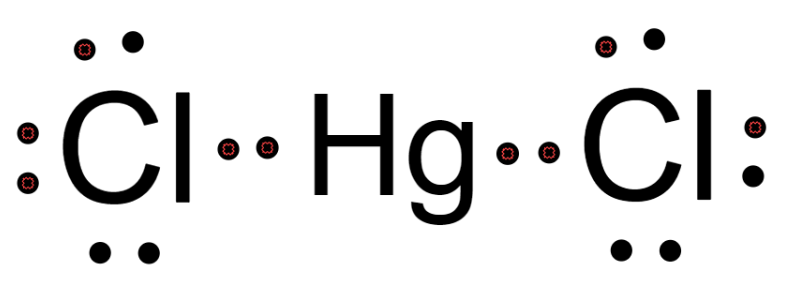
Hence, the correct answer is (1) sp.
Note:
The steric number defines the shape and geometry of the molecule by assigning the position of atoms in such a way that the repulsion between electron pairs will be minimal. For example, a steric number of 2 provides a linear shape in which the bonding electrons are 180 degrees apart.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




