
The hyper conjugative stabilities of tert-butyl cation and $2$-butene, respectively, are due to:
A. $\sigma \to p$ (empty) and $\sigma \to {{\pi }^{*}}$ electron delocalisations.
B. $\sigma \to {{\sigma }^{*}}$ and $\sigma \to \pi $ electron delocalisations.
C. $\sigma \to p$ (filled) and $\sigma \to \pi $ electron delocalisations.
D. $p (filled)\to {{\sigma }^{*}}$ and $\sigma \to {{\pi }^{*}}$ electron delocalisations.
Answer
579.9k+ views
Hint: To find out the correct option for the given question, you have to state what is hyperconjugation and what is the reason behind the hyperconjugation. Try to state about the interaction of the electrons present in different orbitals.
Complete step by step answer:
So, to find the appropriate solution let us first know about what is hyperconjugation.
Hyperconjugation is the stabilising interaction which further results from the interaction of the electrons in a sigma bond, which is denoted by $\sigma $ (usually between a carbon and hydrogen atom or a carbon and carbon atom) with an adjacent empty or partially filled non-bonding p-orbital or an antibonding $\pi $ orbital or filled $\pi $ orbital, to give an extended molecular orbital that increases that stability of the system or compound. So, the hyperconjugation ultimately results in the extended molecular orbital and increases the stability of the compound.
So, we can say that the systems containing a delocalisation in $\sigma \to p$(empty) orbital or $\sigma \to p$(partly filled) orbital or $\sigma \to {{\pi }^{*}}$ orbital or in $\sigma \to \pi $ orbital will tend to give an extended molecular orbital and increase their stability.
Now, let us look on the compounds given in the question and see what type of delocalisation they have.
So, in case of tert-butyl cation, the hyperconjugation is due to the interaction of the electrons in the $\sigma $ bond with the adjacent empty p-orbital.
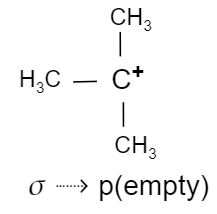
While in case of $2$-butene, the hyperconjugation is due to the interaction of $\sigma $ orbital electrons with the antibonding $\pi $ orbital electrons (${{\pi }^{*}}$).
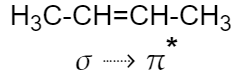
Therefore, the hyper conjugative stabilities of tert-butyl cation and $2$-butene, respectively, are due to $\sigma \to p$ (empty) and $\sigma \to {{\pi }^{*}}$ electron delocalisations.
So, the correct answer is “Option A”.
Note: The stabilisation due to hyperconjugation arises because the orbital interaction leads to the electrons being in a lower energy orbital. The more hyperconjugation there is, the greater the stabilisation of the system. It is also known as “no bond resonance”.
Complete step by step answer:
So, to find the appropriate solution let us first know about what is hyperconjugation.
Hyperconjugation is the stabilising interaction which further results from the interaction of the electrons in a sigma bond, which is denoted by $\sigma $ (usually between a carbon and hydrogen atom or a carbon and carbon atom) with an adjacent empty or partially filled non-bonding p-orbital or an antibonding $\pi $ orbital or filled $\pi $ orbital, to give an extended molecular orbital that increases that stability of the system or compound. So, the hyperconjugation ultimately results in the extended molecular orbital and increases the stability of the compound.
So, we can say that the systems containing a delocalisation in $\sigma \to p$(empty) orbital or $\sigma \to p$(partly filled) orbital or $\sigma \to {{\pi }^{*}}$ orbital or in $\sigma \to \pi $ orbital will tend to give an extended molecular orbital and increase their stability.
Now, let us look on the compounds given in the question and see what type of delocalisation they have.
So, in case of tert-butyl cation, the hyperconjugation is due to the interaction of the electrons in the $\sigma $ bond with the adjacent empty p-orbital.
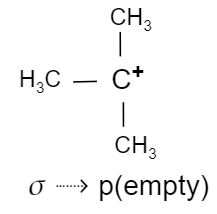
While in case of $2$-butene, the hyperconjugation is due to the interaction of $\sigma $ orbital electrons with the antibonding $\pi $ orbital electrons (${{\pi }^{*}}$).
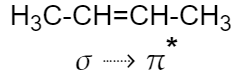
Therefore, the hyper conjugative stabilities of tert-butyl cation and $2$-butene, respectively, are due to $\sigma \to p$ (empty) and $\sigma \to {{\pi }^{*}}$ electron delocalisations.
So, the correct answer is “Option A”.
Note: The stabilisation due to hyperconjugation arises because the orbital interaction leads to the electrons being in a lower energy orbital. The more hyperconjugation there is, the greater the stabilisation of the system. It is also known as “no bond resonance”.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




