
The interparticle force between linear chains in Nylon -6,6 are _________.
A. Hydrogen bonds
B. Covalent bonds
C. Ionic bonds
D. Coordinate bonds
Answer
564.6k+ views
Hint:We know that Nylon-6,6 is a polymer. Polymers are large molecules or macromolecules formed by a chemical combination of a large number of relatively small molecules called monomers. It is part of the polymer family of linear polyamides.
Complete step by step answer:
Here we have to find the interparticle force between linear chains of Nylon-6,6 so for that we have to understand its structure.
Let’s begin with their preparation, Nylon-6,6 is prepared using adipic acid and hexamethylenediamine. This gives us a product as Nylon that has an exact ratio of 1:1 acid to base. This formed Nylon is then dried and heated under vacuum to remove water and they form the polymer. We can also make this by compound that has amine at one end and acid at the other are polymerized to produce a chain with repeating units $\left( { - N{H_2} - \left[ {C{H_2}} \right]n - CO - } \right)x$
So the structure of Nylon-6,6 would look like this.
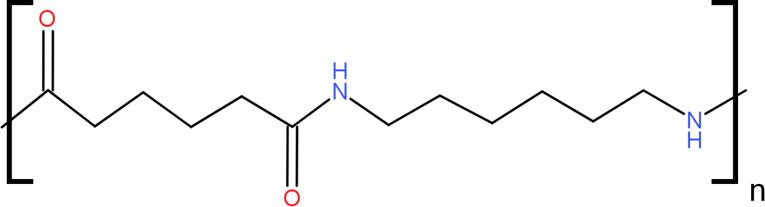
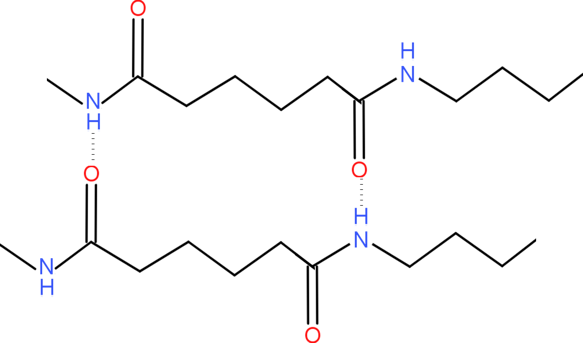
Where n represents the number of repeating chains which makes them as polymers. The polymer is formed by the nitrogen bonded hydrogen of one nylon chain from hydrogen bond with carbonyl oxygens of another nylon chain. Nylon is very strong due to this hydrogen bond between them, they hold them very tightly. The hydrogen bond between them would look like this. We can see the hydrogen bond formed in the picture.
Hence, option (A) is correct.
Note: Apart from Nylon-6,6, Nylon has other polymers too. Such as Nylon 6 which is formed by ring-opening polymerisation, Nylon 510 which is obtained by sebacic and pentamethylene diamine acid also Nylon 1,6 which is produced from nitriles with the help of acid catalysis.
Complete step by step answer:
Here we have to find the interparticle force between linear chains of Nylon-6,6 so for that we have to understand its structure.
Let’s begin with their preparation, Nylon-6,6 is prepared using adipic acid and hexamethylenediamine. This gives us a product as Nylon that has an exact ratio of 1:1 acid to base. This formed Nylon is then dried and heated under vacuum to remove water and they form the polymer. We can also make this by compound that has amine at one end and acid at the other are polymerized to produce a chain with repeating units $\left( { - N{H_2} - \left[ {C{H_2}} \right]n - CO - } \right)x$
So the structure of Nylon-6,6 would look like this.

Where n represents the number of repeating chains which makes them as polymers. The polymer is formed by the nitrogen bonded hydrogen of one nylon chain from hydrogen bond with carbonyl oxygens of another nylon chain. Nylon is very strong due to this hydrogen bond between them, they hold them very tightly. The hydrogen bond between them would look like this. We can see the hydrogen bond formed in the picture.

Hence, option (A) is correct.
Note: Apart from Nylon-6,6, Nylon has other polymers too. Such as Nylon 6 which is formed by ring-opening polymerisation, Nylon 510 which is obtained by sebacic and pentamethylene diamine acid also Nylon 1,6 which is produced from nitriles with the help of acid catalysis.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




