
The ion which is not tetrahedral in shape
$B{{F}_{4}}^{-}$
$N{{H}_{4}}^{+}$
$Xe{{O}_{4}}$
$IC{{l}_{4}}^{-}$
Answer
584.7k+ views
Hint: The 3-D arrangement of atoms in a molecule is known as its molecular geometry. The molecular geometry includes the shape of the molecule as well as the bond length and bond angles of the molecule. All these factors help to determine the position of each atom in the geometry.
Complete step by step solution:
The general formula which we use to calculate the hybridization of a molecule is
Hybridization number =$\dfrac{1}{2}\left[ V+M-C+A \right]$
Here V = the number of valence electrons of the central atom
C = charge on cation
A= charge on anion
M is the number of atoms linked to the central atom
For $B{{F}_{4}}^{-}$
Hybridization number = $\dfrac{1}{2}\left[ 3+4-0+1 \right]=4$
And hybridization will be $s{{p}^{3}}$ and shape is tetrahedral
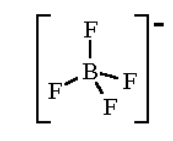
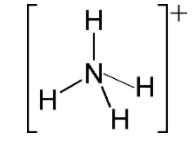
For $N{{H}_{4}}^{+}$
Hybridization number = $\dfrac{1}{2}\left[ 5+4-1+0 \right]=4$
And hybridization will be $s{{p}^{3}}$ and shape is tetrahedral
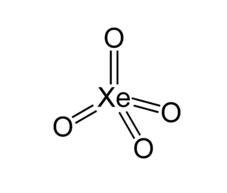
For $Xe{{O}_{4}}$
Xe has 8 unpaired electrons but oxygen is not a monovalent atom, oxygen is a divalent atom and the above rule is only applicable for monovalent atoms. Another way to calculate hybridization is adding sigma bonds to lone pairs.
Xe has no lone pair and form 4 sigma bond: hybridization = $4+0=4$
And hybridization will be $s{{p}^{3}}$ and shape is tetrahedral
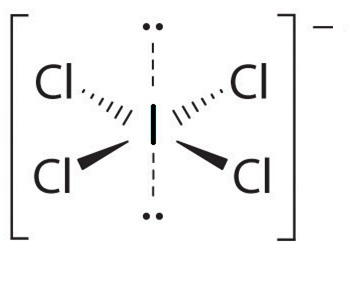
For $IC{{l}_{4}}^{-}$
Hybridization number = $\dfrac{1}{2}\left[ 7+4-0+1 \right]=6$
And hybridization will be $s{{p}^{3}}{{d}^{2}}$ and the shape is square planar.
Hence the correct option is option (D)
Note: The geometry and shape of a molecule can be the same or different as the geometry of the molecule depends on the arrangement of lone pair and bond Pair while the shape of a molecule excludes the lone pair on the central atom.
Complete step by step solution:
The general formula which we use to calculate the hybridization of a molecule is
Hybridization number =$\dfrac{1}{2}\left[ V+M-C+A \right]$
Here V = the number of valence electrons of the central atom
C = charge on cation
A= charge on anion
M is the number of atoms linked to the central atom
For $B{{F}_{4}}^{-}$
Hybridization number = $\dfrac{1}{2}\left[ 3+4-0+1 \right]=4$
And hybridization will be $s{{p}^{3}}$ and shape is tetrahedral

For $N{{H}_{4}}^{+}$
Hybridization number = $\dfrac{1}{2}\left[ 5+4-1+0 \right]=4$
And hybridization will be $s{{p}^{3}}$ and shape is tetrahedral

For $Xe{{O}_{4}}$
Xe has 8 unpaired electrons but oxygen is not a monovalent atom, oxygen is a divalent atom and the above rule is only applicable for monovalent atoms. Another way to calculate hybridization is adding sigma bonds to lone pairs.
Xe has no lone pair and form 4 sigma bond: hybridization = $4+0=4$
And hybridization will be $s{{p}^{3}}$ and shape is tetrahedral

For $IC{{l}_{4}}^{-}$
Hybridization number = $\dfrac{1}{2}\left[ 7+4-0+1 \right]=6$
And hybridization will be $s{{p}^{3}}{{d}^{2}}$ and the shape is square planar.

Hence the correct option is option (D)
Note: The geometry and shape of a molecule can be the same or different as the geometry of the molecule depends on the arrangement of lone pair and bond Pair while the shape of a molecule excludes the lone pair on the central atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




