
The isomeric pair is-
a.Ethane and propane
b.Propane and butane
c.Ethane and butane
d.Butane and \[2 - methylpropane\]
Answer
468.9k+ views
Hint: For a pair of hydrocarbons to be isomeric in nature, the number of carbon atoms in both the hydrocarbons must be the same. Count the number of carbon atoms in hydrocarbons by referring to the prefix of their IUPAC names and identify the pair with the same number of carbon atoms.
Complete step by step answer:
Hydrocarbons are made up of chains of carbon atoms connected to each other through covalent bonds. The bond formation can be in the form of a straight chain where each carbon atom is connected to two other adjacent carbon atoms (except for the terminal carbon atoms) or it can be in a branched form.
The branching of the carbon chain means that two or more straight chains of carbon atoms intersect or get linked to one another at positions other than the terminal carbon atoms.
A straight chain and a branched hydrocarbon can have the same number of carbon atoms and the same molecular formula. Such a pair of hydrocarbons with the same molecular formula but different bond connectivities or different structures are called isomers.
Ethane, propane and butane consist of two, three and four carbon atoms respectively. Since, all of them have a different molecular formula they cannot be called isomers of each other.
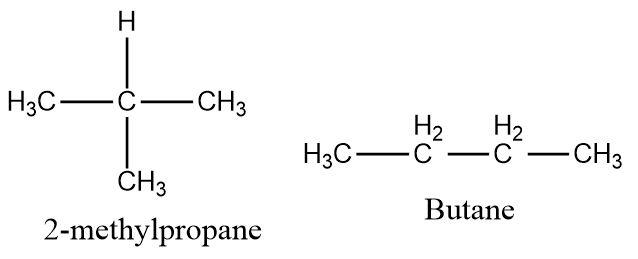
Butane and \[2 - methylpropane\] contain four carbon atoms each and have the same molecular formula \[{C_4}{H_{10}}\] but the structures of these two compounds are different and therefore they form a pair of isomers.
Hence, the correct option is (d).
Note:
The isomers can be of different types like functional group isomers, geometrical isomers or optical isomers. Butane and \[2 - methylpropane\] are structural isomers as they have the same molecular formula and the same number of carbon atoms but differ only in their structures.
Complete step by step answer:
Hydrocarbons are made up of chains of carbon atoms connected to each other through covalent bonds. The bond formation can be in the form of a straight chain where each carbon atom is connected to two other adjacent carbon atoms (except for the terminal carbon atoms) or it can be in a branched form.
The branching of the carbon chain means that two or more straight chains of carbon atoms intersect or get linked to one another at positions other than the terminal carbon atoms.
A straight chain and a branched hydrocarbon can have the same number of carbon atoms and the same molecular formula. Such a pair of hydrocarbons with the same molecular formula but different bond connectivities or different structures are called isomers.
Ethane, propane and butane consist of two, three and four carbon atoms respectively. Since, all of them have a different molecular formula they cannot be called isomers of each other.
Butane and \[2 - methylpropane\] contain four carbon atoms each and have the same molecular formula \[{C_4}{H_{10}}\] but the structures of these two compounds are different and therefore they form a pair of isomers.

Hence, the correct option is (d).
Note:
The isomers can be of different types like functional group isomers, geometrical isomers or optical isomers. Butane and \[2 - methylpropane\] are structural isomers as they have the same molecular formula and the same number of carbon atoms but differ only in their structures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




