
The IUPAC name for the given hydrocarbon is:
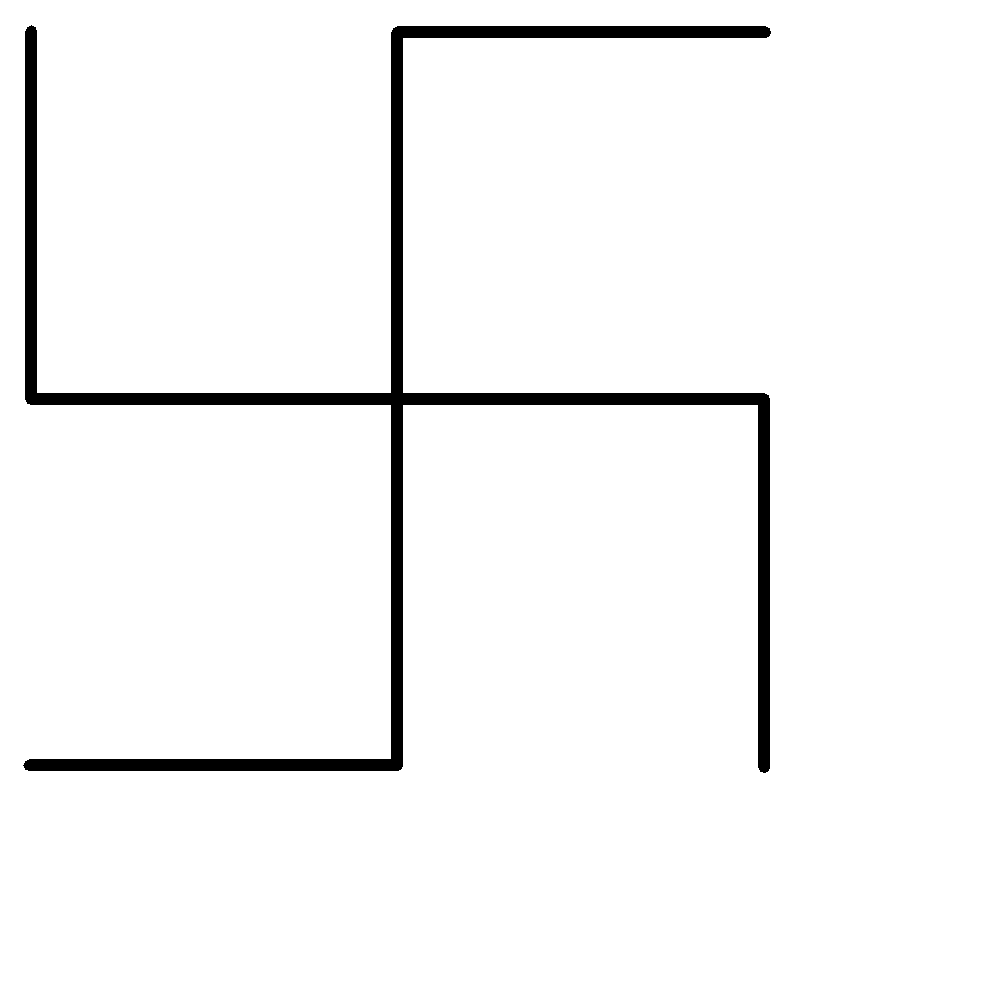
A.Neononane
B.Tetraethyl carbon
C.2-ethylpentane
D.3,3-diethyl pentane

Answer
594k+ views
Hint:
While naming hydrocarbons, look for the longest chain first. The longest chain in the hydrocarbon is the parent chain.
Complete step by step answer:
First of all, let us simplify the structure of the hydrocarbon given above:
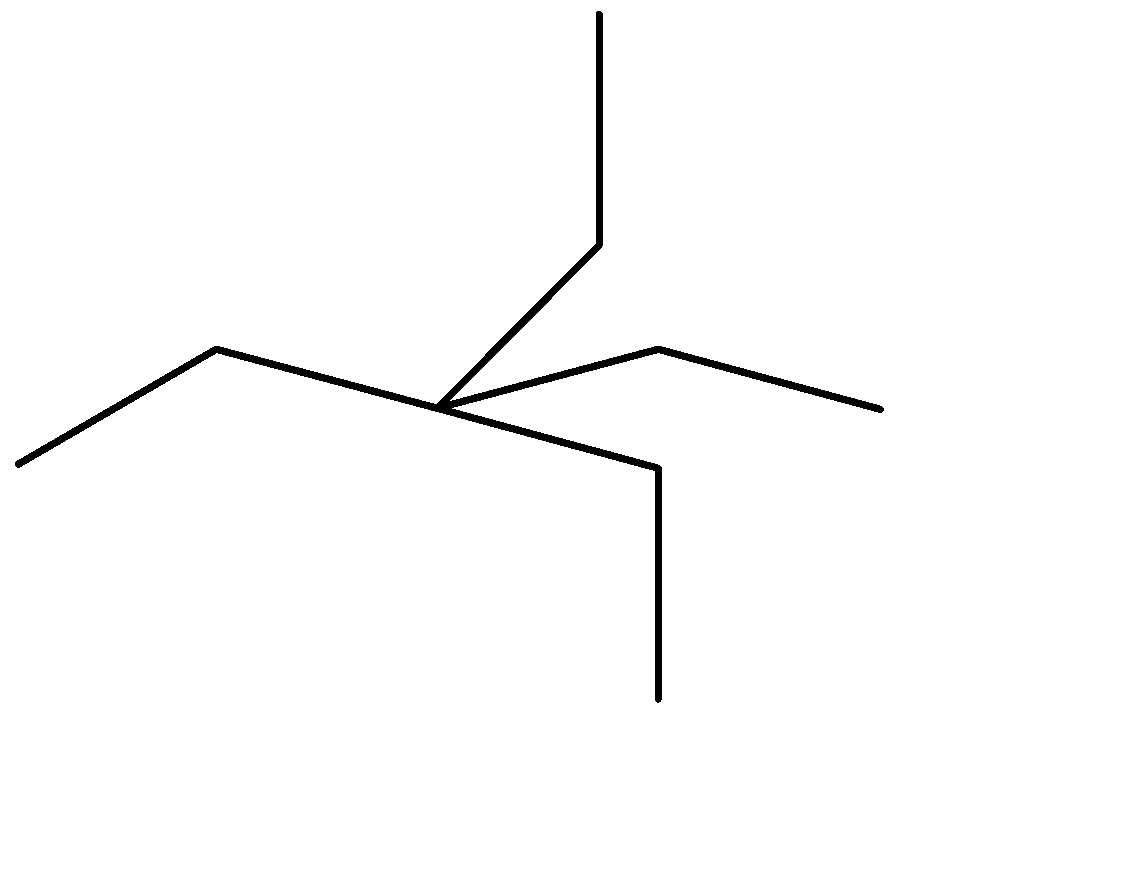
Simplifying it further, we get
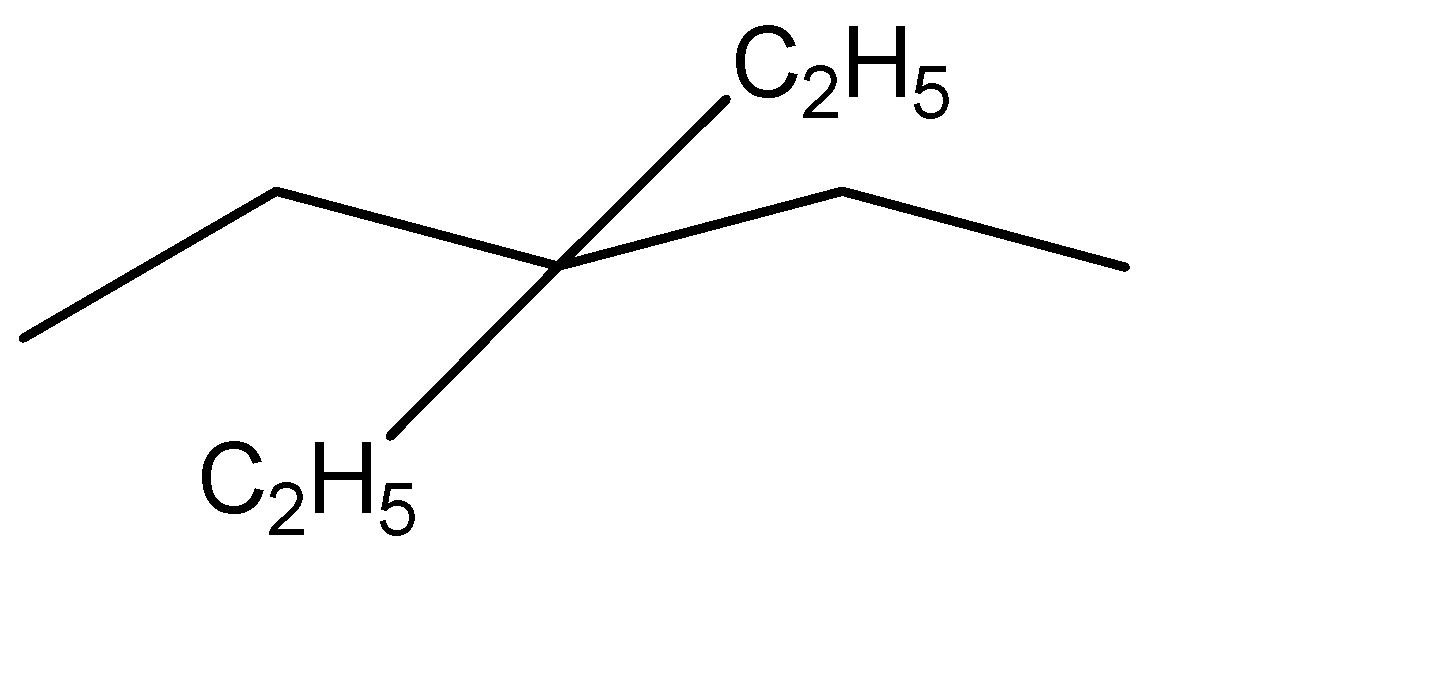
Now that we have the simplified representation of the given hydrocarbon, we can observe:
-The longest single carbon-carbon chain contains 5 carbon atoms.
-There are no double or triple carbon bonds on the longest chain, hence the given organic compound is an alkane.
-The only functional groups to be present on the longest chain are 2 ethyl groups on the third carbon atom from either side.
Now, let us discuss some rules for the nomenclature of such ‘alkanes’
-Find the longest chain of carbon atoms. This will be the parent chain of the compound.
Then identify all the substituents of the functional groups.
-While numbering the functional group, the numbering on the parent chain should be such that it should be closest to the terminal carbon atom.
-If the same functional group is occurring more than once, then while mentioning the functional group in the name of the hydrocarbon, appropriate position numbering should be mentioned. In addition, the number of times the substituent group occurs is indicated by a prefix (di, tri, tetra, etc.).
-If chains of equal length are competing for selection as the parent chain, then the choice goes in series to:
a) the chain which has the greatest number of side chains.
b) the chain whose substituents have the lowest- numbers.
c) the chain having the greatest number of carbon atoms in the smaller side chain.
d)the chain having the least branched side chains.
-Following these rules and observing the hydrocarbon in question, we can say that,
Parent chain has 5 carbon atoms and is hence named as pentane
2 Ethyl groups are present at the third position. His these will be named as 3,3-diethyl
Hence, the IUPAC nomenclature of the given compound is 3,3-diethyl pentane.
Hence, Option D is the correct option.
Note:
The names of the substituents formed by the removal of one hydrogen from the end of the chain is obtained by changing the suffix -ane to -yl.
While naming hydrocarbons, look for the longest chain first. The longest chain in the hydrocarbon is the parent chain.
Complete step by step answer:
First of all, let us simplify the structure of the hydrocarbon given above:

Simplifying it further, we get

Now that we have the simplified representation of the given hydrocarbon, we can observe:
-The longest single carbon-carbon chain contains 5 carbon atoms.
-There are no double or triple carbon bonds on the longest chain, hence the given organic compound is an alkane.
-The only functional groups to be present on the longest chain are 2 ethyl groups on the third carbon atom from either side.
Now, let us discuss some rules for the nomenclature of such ‘alkanes’
-Find the longest chain of carbon atoms. This will be the parent chain of the compound.
Then identify all the substituents of the functional groups.
-While numbering the functional group, the numbering on the parent chain should be such that it should be closest to the terminal carbon atom.
-If the same functional group is occurring more than once, then while mentioning the functional group in the name of the hydrocarbon, appropriate position numbering should be mentioned. In addition, the number of times the substituent group occurs is indicated by a prefix (di, tri, tetra, etc.).
-If chains of equal length are competing for selection as the parent chain, then the choice goes in series to:
a) the chain which has the greatest number of side chains.
b) the chain whose substituents have the lowest- numbers.
c) the chain having the greatest number of carbon atoms in the smaller side chain.
d)the chain having the least branched side chains.
-Following these rules and observing the hydrocarbon in question, we can say that,
Parent chain has 5 carbon atoms and is hence named as pentane
2 Ethyl groups are present at the third position. His these will be named as 3,3-diethyl
Hence, the IUPAC nomenclature of the given compound is 3,3-diethyl pentane.
Hence, Option D is the correct option.
Note:
The names of the substituents formed by the removal of one hydrogen from the end of the chain is obtained by changing the suffix -ane to -yl.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




