
The IUPAC name of dimethyl ether is:
(A) Ethoxymethane
(B) Methoxymethane
(C) Methoxyethane
(D) Ethoxyethane
Answer
537.3k+ views
Hint: Dimethyl ether is an organic compound of the ether family. Ethers are defined as a group of organic compounds in which an oxygen atom is bonded to two alkyl or aryl groups. These two groups could be the same or different. To find the correct IUPAC name we need to draw the structure of dimethyl ether.
Complete step by step solution:
The general formula of ether is \[R - O - R\], where \[R\] is an alkyl group. According to the attached substituent group ethers are classified into two categories;
Symmetrical ethers: In these ethers, the substituent group attached to the oxygen is same or identical.
Asymmetrical ethers: In these ethers, the substituent groups attached to the oxygen are different.
The Nomenclature of ethers is done in two standard ways:
Common nomenclature
IUPAC nomenclature
The chemical formula of dimethyl ether is \[{C_2}{H_6}O\]. The structure for dimethyl ether is:
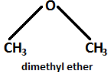
The Common nomenclature of ether is done by naming different alkyl or aryl groups bonded to the oxygen atom on either side in alphabetical order and adding the word ether to it in the end. For example, \[{C_2}{H_5}O{C_6}{H_5}\] will be called ethyl phenyl ether.
In the IUPAC nomenclature, the guidelines are different. According to the rule of IUPAC, naming a substituent group in which more carbon atoms are present is chosen as the parent hydrocarbon. The other substituent group present on the oxygen atom will be named with the prefix “oxy”. For example, \[C{H_3}O{C_2}{H_5}\] will be named as \[1 - methoxyethane\].
In the case of dimethyl ether we can see there are two alkyl groups bonded to the oxygen atom, Hence according to the IUPAC rules substituent group has the same number of carbon atoms. So, it will be called \[Methoxyethane\].
Hence, Option (B) is the correct answer for the given question.
Note: Ethers are less polar compounds as compared to esters, alcohols, or amines because of the inability of oxygen to participate in the hydrogen bonding due to the presence of bulky alkyl groups on both sides. Dimethyl ether is used as a refrigerant and as a solvent at low temperature.
Complete step by step solution:
The general formula of ether is \[R - O - R\], where \[R\] is an alkyl group. According to the attached substituent group ethers are classified into two categories;
Symmetrical ethers: In these ethers, the substituent group attached to the oxygen is same or identical.
Asymmetrical ethers: In these ethers, the substituent groups attached to the oxygen are different.
The Nomenclature of ethers is done in two standard ways:
Common nomenclature
IUPAC nomenclature
The chemical formula of dimethyl ether is \[{C_2}{H_6}O\]. The structure for dimethyl ether is:

The Common nomenclature of ether is done by naming different alkyl or aryl groups bonded to the oxygen atom on either side in alphabetical order and adding the word ether to it in the end. For example, \[{C_2}{H_5}O{C_6}{H_5}\] will be called ethyl phenyl ether.
In the IUPAC nomenclature, the guidelines are different. According to the rule of IUPAC, naming a substituent group in which more carbon atoms are present is chosen as the parent hydrocarbon. The other substituent group present on the oxygen atom will be named with the prefix “oxy”. For example, \[C{H_3}O{C_2}{H_5}\] will be named as \[1 - methoxyethane\].
In the case of dimethyl ether we can see there are two alkyl groups bonded to the oxygen atom, Hence according to the IUPAC rules substituent group has the same number of carbon atoms. So, it will be called \[Methoxyethane\].
Hence, Option (B) is the correct answer for the given question.
Note: Ethers are less polar compounds as compared to esters, alcohols, or amines because of the inability of oxygen to participate in the hydrogen bonding due to the presence of bulky alkyl groups on both sides. Dimethyl ether is used as a refrigerant and as a solvent at low temperature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




