
The IUPAC name of
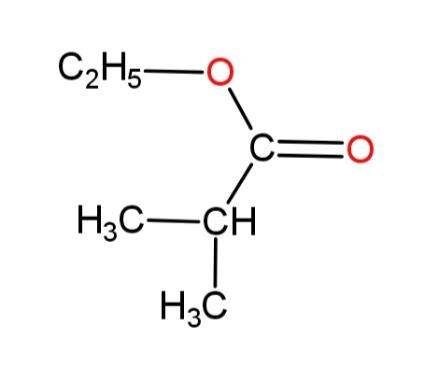
Is:
A. ethoxy methanone
B. ethyl-2-methyl propanoate
C. ethoxypropanone
D. 20methyl ethoxy propanone

Answer
531.9k+ views
Hint: IUPAC stands for international union of pure and applied chemistry. It has made certain rules regarding the naming of organic compounds. The naming depends on the attached functional groups and the number of carbons in the parent chain, which is the longest chain.
Complete answer:
We have been given a structure to find its IUPAC name. We will determine the name through the rules of IUPAC. Some of the rules for IUPAC naming of organic compounds are:
- The functional group is identified, and named according to the specifications of the functional group, like, alkanoic acid for carboxylic acid group, alkanols for alcohols, alkyl alkanoate for esters, and alkoxy alkane for ethers, etc.
Here in the compound we have a carbon attached with two oxygen atoms, which means it is an ester with formula,$RCOO{{R}^{'}}$ , where R is the alkyl group.
- Now the chains are identified in the given compound.
On one side is the ethyl group, while on the other side of the ester is the 3 carbon chain with a methyl branch.
-The name of esters are given as alkyl alkanoate. So here it will be,
The compound has one ethyl chain so ethyl, and the 3 carbon chain has methyl on carbon- 2, so it will be 2 methyl propanoate.
Hence, the IUPAC name of the compound is ethyl-2-methyl propanoate.
So option B is correct.
Note:
In the case of oxygen containing organic compounds, the oxygen chain that is continuous and in the middle is considered a ester functional group. While it can be confused for the keto- carboxylic acids, which have a carbonyl group on beta carbon along with a carboxylic acid group at the end.
Complete answer:
We have been given a structure to find its IUPAC name. We will determine the name through the rules of IUPAC. Some of the rules for IUPAC naming of organic compounds are:
- The functional group is identified, and named according to the specifications of the functional group, like, alkanoic acid for carboxylic acid group, alkanols for alcohols, alkyl alkanoate for esters, and alkoxy alkane for ethers, etc.
Here in the compound we have a carbon attached with two oxygen atoms, which means it is an ester with formula,$RCOO{{R}^{'}}$ , where R is the alkyl group.
- Now the chains are identified in the given compound.
On one side is the ethyl group, while on the other side of the ester is the 3 carbon chain with a methyl branch.
-The name of esters are given as alkyl alkanoate. So here it will be,
The compound has one ethyl chain so ethyl, and the 3 carbon chain has methyl on carbon- 2, so it will be 2 methyl propanoate.
Hence, the IUPAC name of the compound is ethyl-2-methyl propanoate.
So option B is correct.
Note:
In the case of oxygen containing organic compounds, the oxygen chain that is continuous and in the middle is considered a ester functional group. While it can be confused for the keto- carboxylic acids, which have a carbonyl group on beta carbon along with a carboxylic acid group at the end.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




