
The IUPAC name of \[{\left( {{\rm{C}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{2}}}{\rm{CHC}}{{\rm{H}}_{\rm{2}}}{\rm{Br}}\] is:
A.\[{\rm{1 - bromo - 2 - methylpropane}}\]
B.\[{\rm{2 - bromo - 2 - methylpropane}}\]
C.\[{\rm{1 - bromo - 1 - methyl}}\;{\rm{propane}}\]
D.\[{\rm{2 - bromo - 1 - methyl}}\;{\rm{propane}}\]
Answer
583.5k+ views
Hint: The IUPAC nomenclature is a method of naming the organic compounds based on the compound's structural formula. The carbon in which halogen is attached is taken as the parent carbon atom and numbered that carbon.
Step by step answer:
As we all know, the name of an organic compound is derived by first identifying parent hydrocarbons, functional groups, and then finally checking substituents. Hydrocarbon is saturated hydrocarbon if it contains only a single finally bond. The IUPAC name of saturated hydrocarbons is alkanes.
The names of such compounds depend on their structure of a chain and end with a suffix "-ane" and prefix indicating the number of carbon atoms. To identify the IUPAC name of the given compound, first of all, we have to draw the check the number of carbon and substituent positions.
The IUPAC name of \[{\left( {{\rm{C}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{2}}}{\rm{CHC}}{{\rm{H}}_{\rm{2}}}{\rm{Br}}\] is \[{\rm{1 - bromo - 2 - methylpropane}}\].
The structure of this compound is shown below.
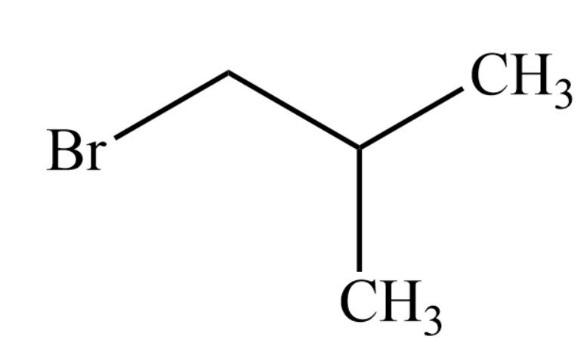
Since the longest carbon chain consists of three carbon atoms, so the parent name will be propane. Also, there is one bromine substituent on the first carbon and the methyl substituent on the second carbon atom. So, the IUPAC name will be 1-Bromo-2-methylpropane.
Hence, the correct option for this question is A that is \[{\rm{1 - bromo - 2 - methylpropane}}\].
Note: Thus, the IUPAC name is based on certain rules that lay the foundation of the hydrocarbons or organic compounds' nomenclature. Before the IUPAC system of nomenclature, the organic compounds were named based on origins.
Step by step answer:
As we all know, the name of an organic compound is derived by first identifying parent hydrocarbons, functional groups, and then finally checking substituents. Hydrocarbon is saturated hydrocarbon if it contains only a single finally bond. The IUPAC name of saturated hydrocarbons is alkanes.
The names of such compounds depend on their structure of a chain and end with a suffix "-ane" and prefix indicating the number of carbon atoms. To identify the IUPAC name of the given compound, first of all, we have to draw the check the number of carbon and substituent positions.
The IUPAC name of \[{\left( {{\rm{C}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{2}}}{\rm{CHC}}{{\rm{H}}_{\rm{2}}}{\rm{Br}}\] is \[{\rm{1 - bromo - 2 - methylpropane}}\].
The structure of this compound is shown below.

Since the longest carbon chain consists of three carbon atoms, so the parent name will be propane. Also, there is one bromine substituent on the first carbon and the methyl substituent on the second carbon atom. So, the IUPAC name will be 1-Bromo-2-methylpropane.
Hence, the correct option for this question is A that is \[{\rm{1 - bromo - 2 - methylpropane}}\].
Note: Thus, the IUPAC name is based on certain rules that lay the foundation of the hydrocarbons or organic compounds' nomenclature. Before the IUPAC system of nomenclature, the organic compounds were named based on origins.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




