
The IUPAC name of methyl acetate is methylethanoate
Answer
588.6k+ views
Hint: Draw the structure of methyl acetate. Now identify the main carbon chain and number the carbon is priority sequence. Remember terminal functional groups are given more preference than normal functional groups. Apply the alphabetical rule to decide whether the methyl group or the ethyl group is substituent.
Complete step-by-step answer:
The International Union of Pure and Applied Chemistry is an international federation of National Adhering Organizations that represents chemists in the individual countries. IUPAC is registered in Zürich; Switzerland and its administrative office is called IUPAC secretariat.
We will now draw the structure of methyl acetate:
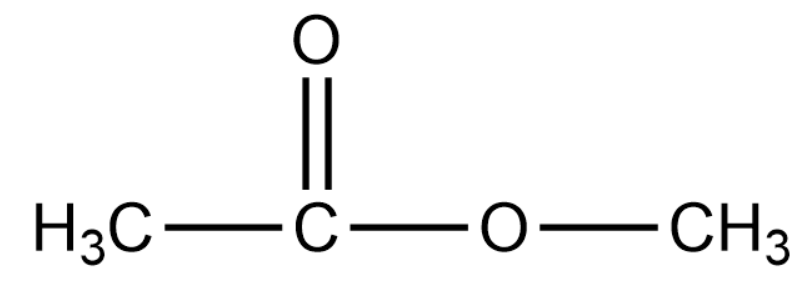
In the above compound, we see that the compound is an ester of ethanoic acid. Ester is a functional group formed on the reaction of a carboxylic acid and an alkanol.
Since methyl group gets attached to ethanoic acid and the letter "m" of methyl is ahead of "e" of ethanoic acid, the substituent group is the methyl group.
The IUPAC name is methylethanoate.
Therefore, the correct answer is option (A).
Note: In the above question we did not have to choose the longest carbon chain and the main functional group as there was only one functional group. However, when we have to choose between the main functional group and longest chain, we give priority to the functional group. The above explanation is shown below:
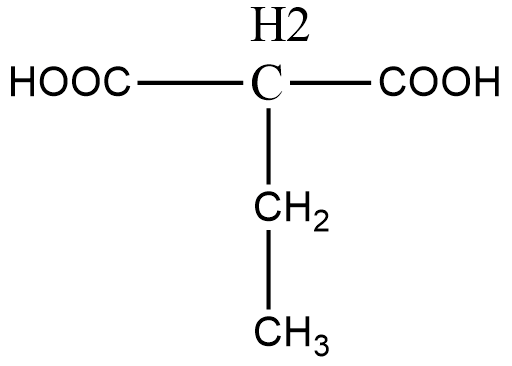
For the above organic compound, the IUPAC name is 2-ethylethan 1,3-dioic acid.
Complete step-by-step answer:
The International Union of Pure and Applied Chemistry is an international federation of National Adhering Organizations that represents chemists in the individual countries. IUPAC is registered in Zürich; Switzerland and its administrative office is called IUPAC secretariat.
We will now draw the structure of methyl acetate:

In the above compound, we see that the compound is an ester of ethanoic acid. Ester is a functional group formed on the reaction of a carboxylic acid and an alkanol.
Since methyl group gets attached to ethanoic acid and the letter "m" of methyl is ahead of "e" of ethanoic acid, the substituent group is the methyl group.
The IUPAC name is methylethanoate.
Therefore, the correct answer is option (A).
Note: In the above question we did not have to choose the longest carbon chain and the main functional group as there was only one functional group. However, when we have to choose between the main functional group and longest chain, we give priority to the functional group. The above explanation is shown below:

For the above organic compound, the IUPAC name is 2-ethylethan 1,3-dioic acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




