
The IUPAC name of tertiary butyl chloride is:
(a) 4-chlororbutane
(b) 2-chlorobutane
(c) 1-chloro-3-methylpropane
(d) 2-chloro-2- methylpropane
Answer
573.6k+ views
Hint: While writing the IUPAC name of any compound, we will first identify the longest carbon chain and we will start numbering from that carbon from which the attached substituent is nearest and while writing the name that name of substituent will be written first which comes first in the alphabetic order along with the carbon number and then add ane, ene or yne if there is -C-C , -C=C or carbon triple bond carbon respectively. Now write the name of the compound.
Complete step by step answer:
The IUPAC stands for the International Union of Pure and Applied Chemistry and it is the method that is used for the naming of the organic or inorganic compounds and the compounds are known by their IUPAC naming all over.
The compound tertiary butyl chloride belongs to the category of alkanes having carbon-carbon single bonds present in it.
In the IUPAC naming of the alkane, the suffix ane is added at the end of the IUPAC name.
Now, writing the IUPAC name of tertiary butyl chloride having the formula as:
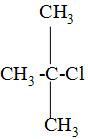
while naming , we have to first choose the longest carbon chain and label that carbon as number 1 from which the substituents that are attached is the closest and mark the labelling on the carbon atoms as 1,2,3---and so on like:
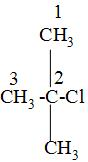
After that, we will look at the attached substituents and write that name of the substituent first which comes first according to the alphabetic order i.e. in the above we can see that the c of chlorine atom comes first then the m of the methyl group i.e. -\[\text{C}{{\text{H}}_{3}}\]group and as the both chlorine and the methyl group is attached to the carbon no-2 so, to dignify their position to which carbon atom they are attached , we will them as 2-chloro and 2-methyl in the IUPAC naming.
Since, the carbon chain consists of three carbon atoms and there are no double and triple bonds in it, so the prefix is prop (C=3) and suffix is ane(C-C) and the carbon chain is named as propane.
So, now the IUPAC name of the compound tertiary butyl chloride is:
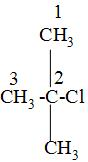
is; 2-chloro-2- methylpropane.
Hence, option(d) is correct.
Note: While labelling the carbon chain keep in mind, the chosen carbon should be the longest in the given compound and in that carbon, chain also start the numbering from that carbon from where the attached functional groups are the nearest
Complete step by step answer:
The IUPAC stands for the International Union of Pure and Applied Chemistry and it is the method that is used for the naming of the organic or inorganic compounds and the compounds are known by their IUPAC naming all over.
The compound tertiary butyl chloride belongs to the category of alkanes having carbon-carbon single bonds present in it.
In the IUPAC naming of the alkane, the suffix ane is added at the end of the IUPAC name.
Now, writing the IUPAC name of tertiary butyl chloride having the formula as:
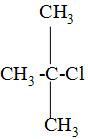
while naming , we have to first choose the longest carbon chain and label that carbon as number 1 from which the substituents that are attached is the closest and mark the labelling on the carbon atoms as 1,2,3---and so on like:
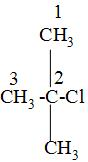
After that, we will look at the attached substituents and write that name of the substituent first which comes first according to the alphabetic order i.e. in the above we can see that the c of chlorine atom comes first then the m of the methyl group i.e. -\[\text{C}{{\text{H}}_{3}}\]group and as the both chlorine and the methyl group is attached to the carbon no-2 so, to dignify their position to which carbon atom they are attached , we will them as 2-chloro and 2-methyl in the IUPAC naming.
Since, the carbon chain consists of three carbon atoms and there are no double and triple bonds in it, so the prefix is prop (C=3) and suffix is ane(C-C) and the carbon chain is named as propane.
So, now the IUPAC name of the compound tertiary butyl chloride is:
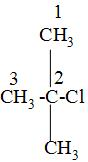
is; 2-chloro-2- methylpropane.
Hence, option(d) is correct.
Note: While labelling the carbon chain keep in mind, the chosen carbon should be the longest in the given compound and in that carbon, chain also start the numbering from that carbon from where the attached functional groups are the nearest
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




