
The IUPAC name of the compound:
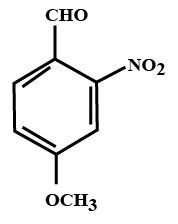
a.) 4-methoxy-2-nitrobenzaldehyde
b.) 2-formyl-3-nitro anisole
c.) 4-methoxy-6-nitro benzaldehyde
d.) 2-formly-5-methoxy nitrobenzene

Answer
598.8k+ views
Hint: Aldehyde is given preference from the above-mentioned functional groups; therefore, numbering will start from the aldehyde group.
Complete step-by-step answer:
The above compound contains three functional groups: aldehyde, nitro and alkoxy groups.
As per the IUPAC norms priority will be given to the aldehyde group. Therefore, aldehyde would be given number 1 position; ether would be given 4 position and in between nitrogen will get the second position.
So, the suffix in the nomenclature will have aldehyde and the prefixes in the nomenclature will be nitro and methoxy. Prefixes will be arranged in alphabetical order.
Prefix for $-OC{{H}_{3}}$ group will be methoxy and for $-N{{O}_{2}}$ group is nitro and the suffix for $-CHO$ group is aldehyde.
Therefore, the IUPAC name of the above compound would be 4-methoxy-2-nitrobenzaldehyde.
The correct option is A.
Note: IUPAC has set certain standards to prioritize functional groups. The decreasing order of priority list is mentioned below:
Carboxylic acid > sulfonic acid > ester > halide > amide > nitrile > aldehyde > ketone > alcohol > thiol > amine
Halides, alkoxides, aside’s and nitro always come as prefixes in naming the compound.
In between alkene and alkyne naming will always end with yen not with end.
The functional group with highest priority will end as a suffix in nomenclature.
And finally, if there are multiple functional groups in a compound and after priority is assessed it comes out that there are more than 1 prefix then naming of the functional group in prefix is written in alphabetical order. For example, in the above problem methoxy was written first than nitro due to their alphabetical order.
Complete step-by-step answer:
The above compound contains three functional groups: aldehyde, nitro and alkoxy groups.
As per the IUPAC norms priority will be given to the aldehyde group. Therefore, aldehyde would be given number 1 position; ether would be given 4 position and in between nitrogen will get the second position.
So, the suffix in the nomenclature will have aldehyde and the prefixes in the nomenclature will be nitro and methoxy. Prefixes will be arranged in alphabetical order.
Prefix for $-OC{{H}_{3}}$ group will be methoxy and for $-N{{O}_{2}}$ group is nitro and the suffix for $-CHO$ group is aldehyde.
Therefore, the IUPAC name of the above compound would be 4-methoxy-2-nitrobenzaldehyde.
The correct option is A.
Note: IUPAC has set certain standards to prioritize functional groups. The decreasing order of priority list is mentioned below:
Carboxylic acid > sulfonic acid > ester > halide > amide > nitrile > aldehyde > ketone > alcohol > thiol > amine
Halides, alkoxides, aside’s and nitro always come as prefixes in naming the compound.
In between alkene and alkyne naming will always end with yen not with end.
The functional group with highest priority will end as a suffix in nomenclature.
And finally, if there are multiple functional groups in a compound and after priority is assessed it comes out that there are more than 1 prefix then naming of the functional group in prefix is written in alphabetical order. For example, in the above problem methoxy was written first than nitro due to their alphabetical order.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




