
The IUPAC name of the compound is:
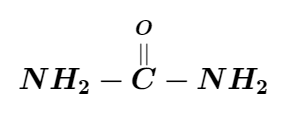
A. Urea
B. Aminomethanamide
C. Diaminomethanal
D. Amino carbamide
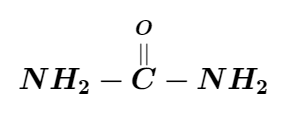
Answer
551.4k+ views
Hint: According to IUPAC (International Union of Pure and Applied Chemistry), whenever we are going to write the IUPAC name of a compound, we have to give numbering first to functional groups or highly substituted carbon (branched carbon). Means lower numbering should be a functional group or highly substituted carbon present in the molecule or compound.
Complete step by step solution:
Before naming the above compound let’s look at a few rules by IUPAC,
-Longest continuous carbon chain is considered for IUPAC nomenclature.
-Then the names of groups attached to the longest continuous carbon chain are considered.
-The location of each of the substituent groups present are designated with an appropriate number and name.
Now let's name the above compound.
The above compound has only one carbon atom along with two amino groups and a carbonyl group. For a single carbon atom, the name used is “meth”. The name of the amino groups are added as a prefix and they are named as “amino”. Since there are two amino groups, we have to use the word “di” before the “amino”. The carbonyl compound present here is an aldehyde and the aldehydes are named in the suffix part as “al”. Since there is no unsaturation of carbon-carbon double bond is present, the compound falls under the category of alkane and it is thus named “al”. So it is named as diaminomethanal. It is more commonly called urea.
So the IUPAC name is diaminomethanal. Hence the correct option is (C).
Note: The IUPAC name of the given compound as per the rule is diaminomethanal. But carbonyl diamide and urea are considered as the most common names of the given compound.
Complete step by step solution:
Before naming the above compound let’s look at a few rules by IUPAC,
-Longest continuous carbon chain is considered for IUPAC nomenclature.
-Then the names of groups attached to the longest continuous carbon chain are considered.
-The location of each of the substituent groups present are designated with an appropriate number and name.
Now let's name the above compound.
The above compound has only one carbon atom along with two amino groups and a carbonyl group. For a single carbon atom, the name used is “meth”. The name of the amino groups are added as a prefix and they are named as “amino”. Since there are two amino groups, we have to use the word “di” before the “amino”. The carbonyl compound present here is an aldehyde and the aldehydes are named in the suffix part as “al”. Since there is no unsaturation of carbon-carbon double bond is present, the compound falls under the category of alkane and it is thus named “al”. So it is named as diaminomethanal. It is more commonly called urea.
So the IUPAC name is diaminomethanal. Hence the correct option is (C).
Note: The IUPAC name of the given compound as per the rule is diaminomethanal. But carbonyl diamide and urea are considered as the most common names of the given compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




