
The IUPAC name of the given compound:

A.$1,1 - $diethyl$ - 2 - $dimethylpentane
B.$4,4 - $dimethyl$ - 5,5 - $ diethylpentane
C.$5,5 - $diethyl$ - 4,4 - $dimethylpentane
D.$3 - $ethyl$ - 4,4 - $dimethylheptane

Answer
579k+ views
Hint: While writing the IUPAC name of a compound, the biggest carbon chain is taken as the parent carbon chain and the substituents are numbered in such a way that all the substituents get the lowest possible numbers. The substituents are written in the name in alphabetical order.
Complete step by step answer:
The given compound is numbered as shown so that the biggest carbon chain is the parent chain:
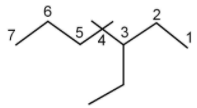
The parent chain has $7$ carbon atoms, hence, the name heptane.
Three substituents are present, $1$ ethyl group at $C - 3$ and $2$ methyl groups at $C - 4$. Alphabetically, ethyl comes before methyl, therefore, the name of the compound will be $3 - $ethyl$ - 4,4 - $dimethylheptane.
Hence option D is correct.
Note:
The names of other compound given in the question will have the following structures,
$1,1 - $diethyl$ - 2 - $dimethylpentane: No structure can be drawn as the IUPAC name is not correct;because for dimethyl the number preceding it should have been $ - 2,2 - $ instead of $ - 2 - $.
$4,4 - $dimethyl$ - 5,5 - $ diethylpentane: The name is not correct because on drawing the structure of the compound the biggest carbon chain is has seven carbon atoms in it as one of the ethyl groups present at $5$ position is a part of the parent chain. The structure can be drawn as,

The correct name of this compound is $3 - $ethyl$ - 4,4 - $dimethylheptane.
$5,5 - $diethyl$ - 4,4 - $dimethylpentane: This name is also incorrect and the structure for this will also be the same as above with the correct name $3 - $ethyl$ - 4,4 - $dimethylheptane.
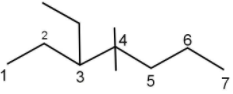
Complete step by step answer:
The given compound is numbered as shown so that the biggest carbon chain is the parent chain:

The parent chain has $7$ carbon atoms, hence, the name heptane.
Three substituents are present, $1$ ethyl group at $C - 3$ and $2$ methyl groups at $C - 4$. Alphabetically, ethyl comes before methyl, therefore, the name of the compound will be $3 - $ethyl$ - 4,4 - $dimethylheptane.
Hence option D is correct.
Note:
The names of other compound given in the question will have the following structures,
$1,1 - $diethyl$ - 2 - $dimethylpentane: No structure can be drawn as the IUPAC name is not correct;because for dimethyl the number preceding it should have been $ - 2,2 - $ instead of $ - 2 - $.
$4,4 - $dimethyl$ - 5,5 - $ diethylpentane: The name is not correct because on drawing the structure of the compound the biggest carbon chain is has seven carbon atoms in it as one of the ethyl groups present at $5$ position is a part of the parent chain. The structure can be drawn as,

The correct name of this compound is $3 - $ethyl$ - 4,4 - $dimethylheptane.
$5,5 - $diethyl$ - 4,4 - $dimethylpentane: This name is also incorrect and the structure for this will also be the same as above with the correct name $3 - $ethyl$ - 4,4 - $dimethylheptane.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




