
The lock and key principle is related to
A. Dark reaction
B. Enzyme action
C. Chemical action
D. Hormonal action
Answer
585.9k+ views
Hint: There are two sites in an enzyme, active site and inactive site. An enzyme attracts substrates to its active site.
Complete answer:
The specific action of an enzyme with a single substrate can be explained using a Lock and Key analogy first postulated. When molecules are involved in a chemical reaction, enzymes act as a catalyst for either breaking them down or building them up into more complex molecules.
1. One characteristic that distinguishes an enzyme from all other types of catalysts is its substrate specificity. Enzymes are proteins that serve the crucial function of “speeding up” chemical reactions.
For example, laundry detergents often come with enzymes that help break down dirt and tough stains on clothing more quickly.
2. Enzymes serve a similar purpose for chemical reactions in an organism’s body, which is to lower the amount of “activation energy” needed for a particular chemical reaction to occur.
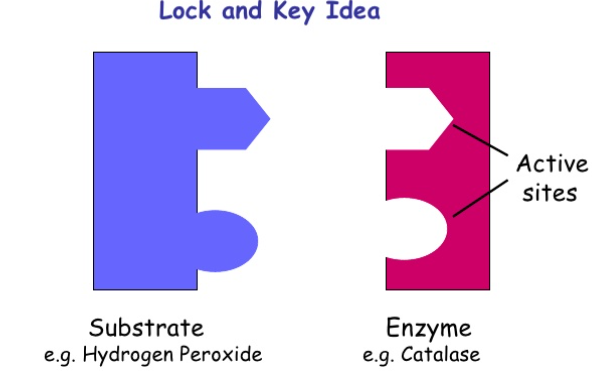
3. During the first stage of a chemical reaction involving enzymes, substrates attach themselves to the active site of an enzyme. Similar to a lock and key, the shape of a substrate determines whether or not it can fit into the active site of a specific enzyme. This is because each enzyme can only catalyze a specific set of chemical reactions. Once the appropriate substrates bind to an enzyme to form an enzyme-substrate complex, a chemical reaction between the substrates can proceed.
4. After the enzyme successfully catalyzes the chemical reaction, the products of the reaction are released and the enzyme is free to repeat the process.
5. The lock and key model for enzyme activity is wrong because it does not account for the intermediate shape of the substrate. In reality, if the situation really was “lock-and-key,” the substrate would get stuck in the enzyme and be unable to move or be released.
6. The enzyme and the substrate are in the same area. Some situations have more than one substrate molecule that the enzyme will change.
So, the correct answer is “Option B”.
Note: Enzymes increase the rate of chemical reactions by reducing the amount of activation energy needed for reactants to start reacting. Enzymes aren’t changed or used up in the reactions they catalyze, so they can be used to speed up the same reaction over and over again.
Complete answer:
The specific action of an enzyme with a single substrate can be explained using a Lock and Key analogy first postulated. When molecules are involved in a chemical reaction, enzymes act as a catalyst for either breaking them down or building them up into more complex molecules.
1. One characteristic that distinguishes an enzyme from all other types of catalysts is its substrate specificity. Enzymes are proteins that serve the crucial function of “speeding up” chemical reactions.
For example, laundry detergents often come with enzymes that help break down dirt and tough stains on clothing more quickly.

2. Enzymes serve a similar purpose for chemical reactions in an organism’s body, which is to lower the amount of “activation energy” needed for a particular chemical reaction to occur.
3. During the first stage of a chemical reaction involving enzymes, substrates attach themselves to the active site of an enzyme. Similar to a lock and key, the shape of a substrate determines whether or not it can fit into the active site of a specific enzyme. This is because each enzyme can only catalyze a specific set of chemical reactions. Once the appropriate substrates bind to an enzyme to form an enzyme-substrate complex, a chemical reaction between the substrates can proceed.
4. After the enzyme successfully catalyzes the chemical reaction, the products of the reaction are released and the enzyme is free to repeat the process.
5. The lock and key model for enzyme activity is wrong because it does not account for the intermediate shape of the substrate. In reality, if the situation really was “lock-and-key,” the substrate would get stuck in the enzyme and be unable to move or be released.
6. The enzyme and the substrate are in the same area. Some situations have more than one substrate molecule that the enzyme will change.
So, the correct answer is “Option B”.
Note: Enzymes increase the rate of chemical reactions by reducing the amount of activation energy needed for reactants to start reacting. Enzymes aren’t changed or used up in the reactions they catalyze, so they can be used to speed up the same reaction over and over again.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




