
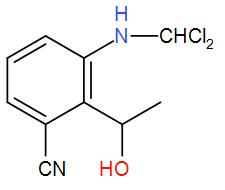
The major product obtained in the following reaction is:
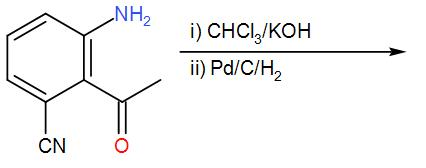
(A)
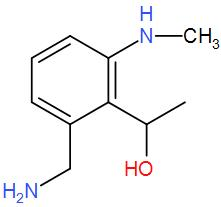
(B)
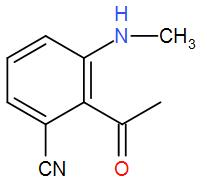
(C)
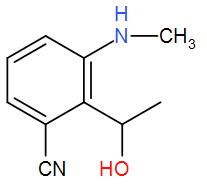
(D)
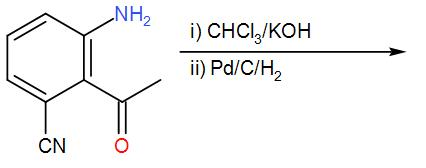




Answer
577.8k+ views
HINT: To solve this, try to recall the working mechanism of the given reagents. Chloroform with alcoholic potassium hydroxide will lead to the formation of isocyanide from the primary amine. Remember that hydrogen, palladium make a strong reducing agent and the presence of carbon will catalyse the reaction.
COMPLETE STEP BY STEP SOLUTION: To answer this question, firstly we need to discuss the working principle of the given reagents.
-Here, a primary amine compound is given to us. When we treat a primary amine with chloroform that is $CHC{{l}_{3}}$ in an alkaline medium in presence of alcohol potassium hydroxide, carbylamines are formed. It is also known as isocyanide and its formation is indicated by a foul smell.
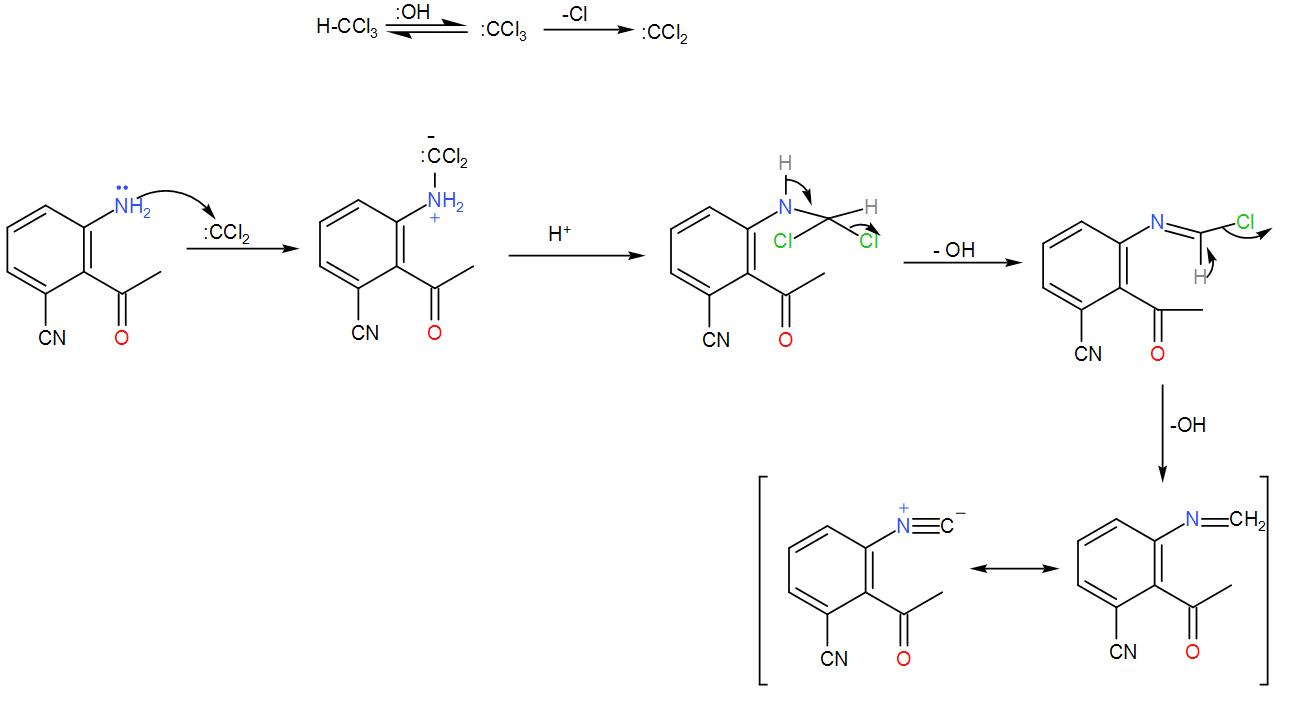
-Now, let us see the reaction mechanism.
Here, at first dichlorocarbene is formed from chloroform by its dehydrohalogenation. The dichlorocarbene is added to the amine and then the base causes dehydrochlorination and eventually leads to the formation of isocyanide. We can write down the reaction as-
Now, to this we have added hydrogen and palladium. These two make a strong reducing agent and carbon catalyses the reaction. The presence of carbon makes reduction of the two groups feasible. This will reduce the cyanide group and give us a primary amine and the isocyanide will give us a secondary amine. Also, the carbonyl carbon is attacked the group is reduced to give alcohol. We can write the reaction as-
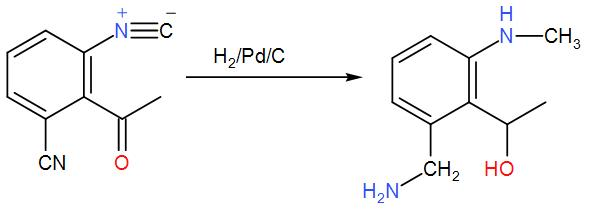
Therefore, we can understand that the correct answer is option-
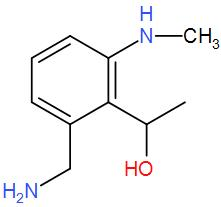
NOTE: We should remember that if primary amine is present, then the reagent chloroform in alkaline medium i.e. in presence of alcoholic potassium hydroxide gives isocyanide. Isocyanide is a foul smelling compound. This is also a test for primary amines as secondary and tertiary amines do not undergo this reaction.
COMPLETE STEP BY STEP SOLUTION: To answer this question, firstly we need to discuss the working principle of the given reagents.
-Here, a primary amine compound is given to us. When we treat a primary amine with chloroform that is $CHC{{l}_{3}}$ in an alkaline medium in presence of alcohol potassium hydroxide, carbylamines are formed. It is also known as isocyanide and its formation is indicated by a foul smell.
-Now, let us see the reaction mechanism.
Here, at first dichlorocarbene is formed from chloroform by its dehydrohalogenation. The dichlorocarbene is added to the amine and then the base causes dehydrochlorination and eventually leads to the formation of isocyanide. We can write down the reaction as-

Now, to this we have added hydrogen and palladium. These two make a strong reducing agent and carbon catalyses the reaction. The presence of carbon makes reduction of the two groups feasible. This will reduce the cyanide group and give us a primary amine and the isocyanide will give us a secondary amine. Also, the carbonyl carbon is attacked the group is reduced to give alcohol. We can write the reaction as-

Therefore, we can understand that the correct answer is option-

NOTE: We should remember that if primary amine is present, then the reagent chloroform in alkaline medium i.e. in presence of alcoholic potassium hydroxide gives isocyanide. Isocyanide is a foul smelling compound. This is also a test for primary amines as secondary and tertiary amines do not undergo this reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




