
The mechanism (using the arrow notation) of the following reaction:\[\text{C}{{\text{H}}_{2}}\text{ = C}{{\text{H}}_{2}}\text{ }\xrightarrow{{{\text{H}}_{3}}{{\text{O}}^{+}}}\text{ C}{{\text{H}}_{3}}\text{ - CH}_{2}^{+}\text{ + }{{\text{H}}_{2}}\text{O}\]
Answer
582.9k+ views
Hint: For this problem, we have to study the action of electrophile and nucleophile on the molecule. Firstly, the breaking of the double bond will take place after it. The addition of hydrogen will take place from the hydronium ion to the ethene molecule.
Complete step by step solution:
- In the given question, we have to explain the mechanism of the given reaction by using the arrow notation.
- As we can see that the hydronium ion is used in the reaction which is acidic and used as a catalyst, so the reaction is known as an acid-catalyzed reaction.
- Now, firstly the breaking of the double bond in the ethene molecules will take place due to which the positive and negative will come on first and second atom respectively as shown below:
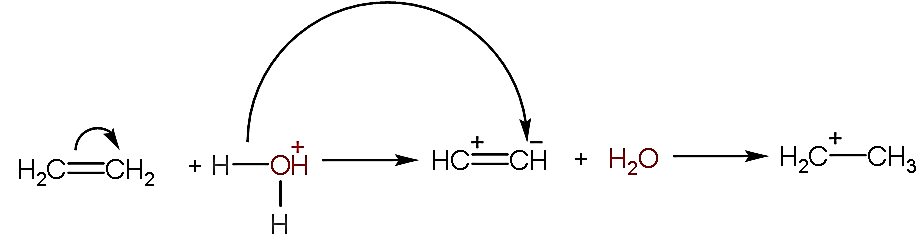
- After the breaking of the bond, one hydrogen atom from hydronium ion will separate and attach with the negative charge of the ethene.
- Now, the product formed will release water as a by-product along with it, it yields ethane ion.
- Now, in the given reaction mechanism, the hydrogen atom is the electrophile because it can accept the lone pair of electrons.
- Whereas the water molecule formed is the nucleophile because it has an extra pair of electrons to donate to the electrophile i.e. ethane ion.
Therefore, the reaction is acid-catalyzed and forms water and ethane ions.
Note: Catalyst in the reaction is used to increase the rate of reaction so that the product can be obtained in very less time but it does not participate in the reaction and also does not alter the product of the reaction.
Complete step by step solution:
- In the given question, we have to explain the mechanism of the given reaction by using the arrow notation.
- As we can see that the hydronium ion is used in the reaction which is acidic and used as a catalyst, so the reaction is known as an acid-catalyzed reaction.
- Now, firstly the breaking of the double bond in the ethene molecules will take place due to which the positive and negative will come on first and second atom respectively as shown below:

- After the breaking of the bond, one hydrogen atom from hydronium ion will separate and attach with the negative charge of the ethene.
- Now, the product formed will release water as a by-product along with it, it yields ethane ion.
- Now, in the given reaction mechanism, the hydrogen atom is the electrophile because it can accept the lone pair of electrons.
- Whereas the water molecule formed is the nucleophile because it has an extra pair of electrons to donate to the electrophile i.e. ethane ion.
Therefore, the reaction is acid-catalyzed and forms water and ethane ions.
Note: Catalyst in the reaction is used to increase the rate of reaction so that the product can be obtained in very less time but it does not participate in the reaction and also does not alter the product of the reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




