
The melting points and solubility in water of amino acids are generally higher than of corresponding halo acids.
Answer
587.1k+ views
Hint:Amino acids exist as zwitterion which is more stable as compared to normal amino acid. Moreover, halo acids are halogen derivative of carboxylic acid Eg.
Complete step by step answer:
We know that amino acids are organic compounds containing amine $\left( { - N{H_2}} \right)$ and carboxyl $\left( { - COOH} \right)$ functional groups. It’s structure is as follow: -
Amino acids are classified as $ \propto ,B,r$ amino acids depending upon position of $ - N{H_2}$ group $w.r.t. - COOH$ group, amino acids combine to form proteins. About 20 amino acids are found in proteins and $6$ amino acids are found in special tissue and deficiency of any one amino acid can cause harm to our body.
Halo acids are any halogen derivative of carboxylic acid $\left[ {eg.{\text{ }}2 - chloro{\text{ }}carboxylic{\text{ }}acid} \right]$.
Now, according to the question, we have given the reason for high melting point and solubility in water of amino acids as compared to halo acids. The amino acids exist as zwitterion in the aqueous solution. zwitterion is the ion that can act both as cation and anion. zwitterion of amino acid is
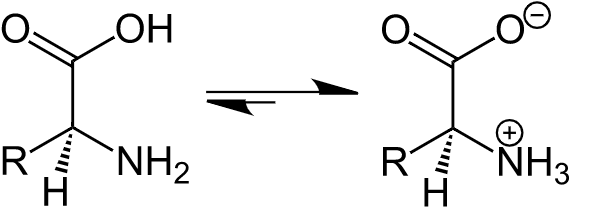
Due to zwitterion, it shows dipole salt nature which is more stable than halo acids. Hence the melting point of amino acids is higher than that of halo acids which do not show any such dipolar nature.
Moreover, we also know that “like dissolves like” i.e. the dipolar substance is easily dissolvable in dipolar solvent. We know that water shows dipolar nature due to charge separation ${H^ + }O{H^ - }$ and we have also discussed that amino acids also show dipolar nature. Hence according to the “like dissolve like” principle, we can say that amino acid is more soluble in water than halo acids.
Note:
In halo acids, no charge separation takes place and hence it is a non-polar substance/salt. Moreover, in acidic solution, amino acid exists as cation and in basic solution it exists as anion. At neutral $pH$, amino acids exist as zwitterions and neither migrate towards cathode nor towards anode. This $pH$ value is called isoelectric point.
Complete step by step answer:
We know that amino acids are organic compounds containing amine $\left( { - N{H_2}} \right)$ and carboxyl $\left( { - COOH} \right)$ functional groups. It’s structure is as follow: -
Amino acids are classified as $ \propto ,B,r$ amino acids depending upon position of $ - N{H_2}$ group $w.r.t. - COOH$ group, amino acids combine to form proteins. About 20 amino acids are found in proteins and $6$ amino acids are found in special tissue and deficiency of any one amino acid can cause harm to our body.
Halo acids are any halogen derivative of carboxylic acid $\left[ {eg.{\text{ }}2 - chloro{\text{ }}carboxylic{\text{ }}acid} \right]$.
Now, according to the question, we have given the reason for high melting point and solubility in water of amino acids as compared to halo acids. The amino acids exist as zwitterion in the aqueous solution. zwitterion is the ion that can act both as cation and anion. zwitterion of amino acid is

Due to zwitterion, it shows dipole salt nature which is more stable than halo acids. Hence the melting point of amino acids is higher than that of halo acids which do not show any such dipolar nature.
Moreover, we also know that “like dissolves like” i.e. the dipolar substance is easily dissolvable in dipolar solvent. We know that water shows dipolar nature due to charge separation ${H^ + }O{H^ - }$ and we have also discussed that amino acids also show dipolar nature. Hence according to the “like dissolve like” principle, we can say that amino acid is more soluble in water than halo acids.
Note:
In halo acids, no charge separation takes place and hence it is a non-polar substance/salt. Moreover, in acidic solution, amino acid exists as cation and in basic solution it exists as anion. At neutral $pH$, amino acids exist as zwitterions and neither migrate towards cathode nor towards anode. This $pH$ value is called isoelectric point.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




