
The molecular formula of ammonium sulphate is _______?
(A) ${{\left( \text{N}{{\text{H}}_{4}} \right)}_{2}}\text{S}{{\text{O}}_{4}}$
(B) \[\text{N}{{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}\]
(C) \[\text{N}{{\text{H}}_{4}}\text{S}{{\text{O}}_{4}}\]
(D) none of these
Answer
534k+ views
Hint: Ammonium sulphate is a salt containing ammonium cation and sulphate anion. The ammonium ion carries a +1 charge while sulphate ion carries a -2 charge. So, two ammonium ions will be needed to form neutral salt.
Complete step by step solution: The ammonium sulphate is produced by the treatment of sulphuric acid with ammonia gas. It is an acid-base reaction in which ammonia acts as a base and sulphuric acid acts as a strong acid. So, the acid will lose its proton which will then be accepted by the base and they form a neutral salt molecule.
The reaction involved is shown below:
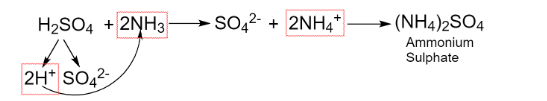
The salt formed is ammonium sulphate which is composed of two molecules of ammonium ions and one molecule of sulphate ion to neutralize the overall charge of the compound.
Additional information: The ammonium sulphate salt has many applications in real life. It is used in most soil fertilizers to alkaline the soil pH and provides the nitrogen content which is very important for the production of the plant.
Hence, the correct option is (A) ${{\left( \text{N}{{\text{H}}_{4}} \right)}_{2}}\text{S}{{\text{O}}_{4}}$.
Note: Ammonium sulphate is an ionic salt and it easily dissolves in water. It is a slightly acidic salt because it is made by the reaction of a strong acid - ${{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$ (sulphuric acid) and a weak base - $\text{N}{{\text{H}}_{3}}$ (ammonia).
Complete step by step solution: The ammonium sulphate is produced by the treatment of sulphuric acid with ammonia gas. It is an acid-base reaction in which ammonia acts as a base and sulphuric acid acts as a strong acid. So, the acid will lose its proton which will then be accepted by the base and they form a neutral salt molecule.
The reaction involved is shown below:
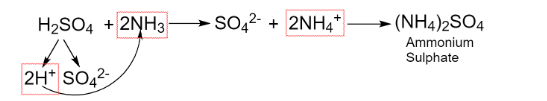
The salt formed is ammonium sulphate which is composed of two molecules of ammonium ions and one molecule of sulphate ion to neutralize the overall charge of the compound.
Additional information: The ammonium sulphate salt has many applications in real life. It is used in most soil fertilizers to alkaline the soil pH and provides the nitrogen content which is very important for the production of the plant.
Hence, the correct option is (A) ${{\left( \text{N}{{\text{H}}_{4}} \right)}_{2}}\text{S}{{\text{O}}_{4}}$.
Note: Ammonium sulphate is an ionic salt and it easily dissolves in water. It is a slightly acidic salt because it is made by the reaction of a strong acid - ${{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$ (sulphuric acid) and a weak base - $\text{N}{{\text{H}}_{3}}$ (ammonia).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




