
The molecular formula of dithionic acid is:
(A) \[{H_2}{S_2}{O_7}\]
(B) \[{H_2}{S_2}{O_6}\]
(C) \[{H_2}{S_2}{O_5}\]
(D) \[{H_2}S{O_5}\]
Answer
569.4k+ views
Hint: In order to determine the molecular formula of dithionic acid, we must know about the structure of dithionic acid. Dithionic acid is said to be an oxo acid of Sulphur. It is diprotic in nature. It will act as mild oxidizing as well as mild reducing agent.
Complete step by step answer:
The free acid of Dithionic acid is not known. It is always present in solution form. Dithionic acids are diprotic in nature. The salts of Dithionic acid are known as the Dithionates. Dithionates are soluble in water. They are the mild reducing as well as the mild oxidizing agent. The Dithionate will be having a structure similar to Ethane.
The structure of Dithionic acid is given below:
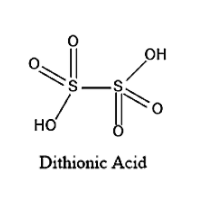
On seeing the above given structure, we can say that the Molecular formula of Dithionic acid is \[{H_2}{S_2}{O_6}\].
Discussing option, (A), the formula \[{H_2}{S_2}{O_7}\], suggests us pyrosulfuric acid.
Discussing option (C), the formula \[{H_2}{S_2}{O_5}\], suggests hyposulfurous acid.
Discussing option (D), the formula \[{H_2}S{O_5}\] suggests Peroxymonosulfuric acid or Caro’s Acid.
Therefore, the correct answer is option (B) \[{H_2}{S_2}{O_6}\].
Additional information:
We can prepare Dithionates by the following methods:
Dithionite can be prepared by the oxidation of sulphite, i.e. the oxidation state changes from +4 to +5. It can also be prepared by oxidizing aqueous solution of Sulphur dioxide with manganese dioxide.
\[2Mn{O_2} + 3S{O_2} \to Mn{S_2}{O_6} + MnS{O_4}\]
Barium dithionate solution on treatment with sulfuric acid will give Dithionic acid.
\[Ba{S_2}{O_6}(aq) + {H_2}S{O_4}(aq) \to {H_2}{S_2}{O_6}(aq) + BaS{O_4}(s) \downarrow \]
Note: Sulphur has various oxoacids. They are:
- Sulphuric acid
- Sulphurous acid
- Permono Sulphuric acid
- Perdi Sulphuric acid
- Thiosulphuric acid
- Dithionic acid
- Pyrosulphuric acid
Complete step by step answer:
The free acid of Dithionic acid is not known. It is always present in solution form. Dithionic acids are diprotic in nature. The salts of Dithionic acid are known as the Dithionates. Dithionates are soluble in water. They are the mild reducing as well as the mild oxidizing agent. The Dithionate will be having a structure similar to Ethane.
The structure of Dithionic acid is given below:

On seeing the above given structure, we can say that the Molecular formula of Dithionic acid is \[{H_2}{S_2}{O_6}\].
Discussing option, (A), the formula \[{H_2}{S_2}{O_7}\], suggests us pyrosulfuric acid.
Discussing option (C), the formula \[{H_2}{S_2}{O_5}\], suggests hyposulfurous acid.
Discussing option (D), the formula \[{H_2}S{O_5}\] suggests Peroxymonosulfuric acid or Caro’s Acid.
Therefore, the correct answer is option (B) \[{H_2}{S_2}{O_6}\].
Additional information:
We can prepare Dithionates by the following methods:
Dithionite can be prepared by the oxidation of sulphite, i.e. the oxidation state changes from +4 to +5. It can also be prepared by oxidizing aqueous solution of Sulphur dioxide with manganese dioxide.
\[2Mn{O_2} + 3S{O_2} \to Mn{S_2}{O_6} + MnS{O_4}\]
Barium dithionate solution on treatment with sulfuric acid will give Dithionic acid.
\[Ba{S_2}{O_6}(aq) + {H_2}S{O_4}(aq) \to {H_2}{S_2}{O_6}(aq) + BaS{O_4}(s) \downarrow \]
Note: Sulphur has various oxoacids. They are:
- Sulphuric acid
- Sulphurous acid
- Permono Sulphuric acid
- Perdi Sulphuric acid
- Thiosulphuric acid
- Dithionic acid
- Pyrosulphuric acid
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




