
What will be the molecular formula of the bond line structure? And how?
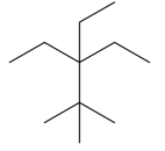

Answer
573.3k+ views
Hint: The concept of bond line formulas is to be used in this question. In the given compound, there are carbons that have methyl and ethyl groups attached to the sides and these contribute to increasing the molar mass of the compound.
Complete step by step answer:
In order to answer our question, we need to learn about the bond line formula for organic compounds. Now, carbon has a valency of 4. That means, one carbon can attach with itself 4 other groups or atoms, so that it’s octet gets filled. This results in the cast range of compounds in the field of organic chemistry. Now, due to the excessive number of compounds, there are some compounds which are very complex or large and it is very time consuming to draw structures of them. Moreover, they will look very messy on paper. So, bond line formulas are preferred. Bond line structures are very simple to draw and understand the structure of very large compounds of organic chemistry. These days, the two dimensional formulae of the organic compounds are also shown with the help of bond line notations in which the bonds are shown with the help of lines only. For example, a single line (-) represents a single C to C bond ; a double line (=) indicates a double C to C bond while a C to C triple bond is shown by a triple line ($\equiv $). For example,
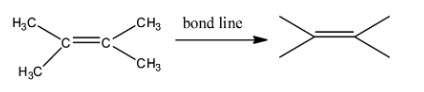
Here the four terminal lines represent the methyl groups. Each and every ‘edge’ represents a carbon atom. Similarly in this way, we can represent other complex carbon compounds. Now, let us come to our question. We have been given the compound :
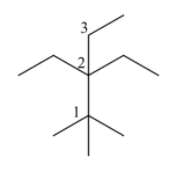
Now, let us name it from carbon 1 to carbon 3. There are 3 methyl groups attached in carbon 1, 2 ethyl groups present in carbon 2 and 1 methyl group in carbon 3. So the molecular formula will be: ${{(C{{H}_{3}})}_{3}}C-{{({{C}_{2}}{{H}_{6}})}_{2}}C-C{{H}_{2}}-C{{H}_{3}}$, or ${{C}_{11}}{{H}_{24}}$.
Note: It is to be noted that the bond line formula is for drawing 2D organic structures. It doesn't give us any idea about whether the bond is directed inside the plane or outside the plane. However, bond line formulas can give us an idea about steric hindrance.
Complete step by step answer:
In order to answer our question, we need to learn about the bond line formula for organic compounds. Now, carbon has a valency of 4. That means, one carbon can attach with itself 4 other groups or atoms, so that it’s octet gets filled. This results in the cast range of compounds in the field of organic chemistry. Now, due to the excessive number of compounds, there are some compounds which are very complex or large and it is very time consuming to draw structures of them. Moreover, they will look very messy on paper. So, bond line formulas are preferred. Bond line structures are very simple to draw and understand the structure of very large compounds of organic chemistry. These days, the two dimensional formulae of the organic compounds are also shown with the help of bond line notations in which the bonds are shown with the help of lines only. For example, a single line (-) represents a single C to C bond ; a double line (=) indicates a double C to C bond while a C to C triple bond is shown by a triple line ($\equiv $). For example,

Here the four terminal lines represent the methyl groups. Each and every ‘edge’ represents a carbon atom. Similarly in this way, we can represent other complex carbon compounds. Now, let us come to our question. We have been given the compound :

Now, let us name it from carbon 1 to carbon 3. There are 3 methyl groups attached in carbon 1, 2 ethyl groups present in carbon 2 and 1 methyl group in carbon 3. So the molecular formula will be: ${{(C{{H}_{3}})}_{3}}C-{{({{C}_{2}}{{H}_{6}})}_{2}}C-C{{H}_{2}}-C{{H}_{3}}$, or ${{C}_{11}}{{H}_{24}}$.
Note: It is to be noted that the bond line formula is for drawing 2D organic structures. It doesn't give us any idea about whether the bond is directed inside the plane or outside the plane. However, bond line formulas can give us an idea about steric hindrance.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




