
The molecule $B{F_3}$ and $N{F_3}$ both are covalent compounds but $B{F_3}$ is non-polar and $N{F_3}$ is polar, the reason is that:
(A) Boron is a metal and nitrogen is a gas in uncombined state
(B) $B{F_3}$ bond have no dipole moment whereas $N{F_3}$ bond have dipole moment
(C) Atomic size of boron is smaller than that of nitrogen
(D) $B{F_3}$ is symmetrical molecule whereas $N{F_3}$ is unsymmetrical
Answer
570k+ views
Hint: Both boron trifluoride, $B{F_3}$ and nitrogen trifluoride, $N{F_3}$are formed by covalent bonding that means the bonds in these compounds are formed by sharing of one or two pairs of valence, electrons. Covalent bonding is very common in compounds formed by combining two non-metals.
Complete step by step solution:
The covalent compounds can be polar or non-polar, depending upon the polarity of bonds involved in these compounds.
In polar covalent compounds, the bonding present is of polar covalent bonding. The bond is formed in two different atoms which have different electronegativity that means there is an unequal sharing of electrons during bond formation between two atoms. The unequal sharing of electrons leads to the development of fractional positive and negative charge on the atoms in a bond and the bond is said to become polar as in $N{F_3}$.
In non-polar compounds, the bonding is a non-polar type that means the electrons are shared between bonded atoms equally. Usually, the electronegativity difference between two bonded atoms in a non-polar bond is less than 0.5. The formation of non-polar covalent bonds may take place if bonded atoms in a polar covalent bond cancel the charges on them by arranging accordingly.
Polarity and non-polarity of molecules depend upon their dipole a moment. Let us first understand the dipole moment of a molecule.
During covalent bond formation, when there is an unequal sharing of electrons between atoms, the separation of charges takes place between atoms resulting in a dipole moment. The dipole moment is denoted as $\mu$ and measured in the Debye unit. The shape of a molecule with dipole moment and the polarity of its bonds are the determining factors for deciding the overall polarity of that molecule. The molecule may contain a polar bond but, the molecule may not be polar.
Let us now understand the structure and bonding in both $B{F_3}$ and $N{F_3}$.
The molecule of $B{F_3}$ contains 1 boron atom and 3 fluorine atoms containing 3 lone pairs of electrons on each fluorine atom. The structure of $B{F_3}$ is given below.
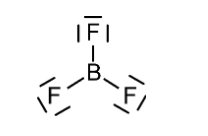
Also according to VSEPR, the $B{F_3}$ molecule has trigonal planar geometry and each $F - B - F$ is of 120°. The $B{F_3}$ molecule is highly symmetrical due to its geometry. Also, the dipole moment of a molecule is a vector quantity that means it requires both magnitude and direction. The three bonds in $B{F_3}$ are polar and they have dipole moments as shown below.
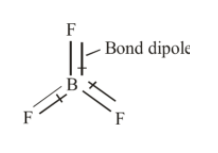
The bond dipole moments in $B{F_3}$ are cancelled due to its symmetrical geometry resulting in a net dipole moment of $B{F_3}$ equal to zero. Hence, $B{F_3}$ is non-polar.
The molecule of $N{F_3}$ contains 1 nitrogen atom and 3 fluorine atoms containing 1 lone pair of electrons on nitrogen atom. The structure of $N{F_3}$ is given below.
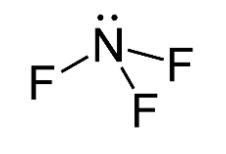
In the molecule $N{F_3}$, three fluorine atoms one electron each to form covalent bonds with nitrogen atoms and nitrogen atom is left with a lone pair of electrons. The repulsion between lone pair of electrons on nitrogen and bond pairs of electrons $N{F_3}$ molecule leads to bent shape of molecule and the resulting geometry is trigonal pyramidal. Hence, the three dipoles on $N - F$ bond originate towards downward direction and one dipole is in upward direction because of lone pair of electrons. The unsymmetrical geometry of $N{F_3}$ molecule causes a non-zero dipole moment in the molecule.
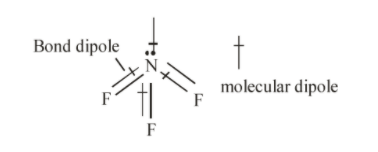
The molecule $B{F_3}$ and $N{F_3}$ both are covalent compounds but $B{F_3}$ is non-polar and $N{F_3}$ is polar, the reason is that $B{F_3}$ is symmetrical molecule whereas $N{F_3}$ is unsymmetrical.
Hence, the correct answer is option (D).
Note: The compound $N{F_3}$is polar as the sharing of valence electrons between atoms in the compound does not take place equally. On the other hand, $B{F_3}$is a polar compound as electrons are shared equally by atoms in a molecule.
The dipole moment is the product of magnitude of the charge and the distance between the centres of positive and negative charges.
Therefore, mathematically dipole moment can be expressed as,
$Dipole\; Moment = charge \times distance\;separation$
$\mu = \delta \times d$
Here, $\mu $is the dipole moment of a bond, $\delta $ represents the magnitude of partial charges ${\delta ^ + }$ and ${\delta ^ - }$. The letter d represents the distance between partial charges ${\delta ^ + }$ and ${\delta ^ - }$.
Complete step by step solution:
The covalent compounds can be polar or non-polar, depending upon the polarity of bonds involved in these compounds.
In polar covalent compounds, the bonding present is of polar covalent bonding. The bond is formed in two different atoms which have different electronegativity that means there is an unequal sharing of electrons during bond formation between two atoms. The unequal sharing of electrons leads to the development of fractional positive and negative charge on the atoms in a bond and the bond is said to become polar as in $N{F_3}$.
In non-polar compounds, the bonding is a non-polar type that means the electrons are shared between bonded atoms equally. Usually, the electronegativity difference between two bonded atoms in a non-polar bond is less than 0.5. The formation of non-polar covalent bonds may take place if bonded atoms in a polar covalent bond cancel the charges on them by arranging accordingly.
Polarity and non-polarity of molecules depend upon their dipole a moment. Let us first understand the dipole moment of a molecule.
During covalent bond formation, when there is an unequal sharing of electrons between atoms, the separation of charges takes place between atoms resulting in a dipole moment. The dipole moment is denoted as $\mu$ and measured in the Debye unit. The shape of a molecule with dipole moment and the polarity of its bonds are the determining factors for deciding the overall polarity of that molecule. The molecule may contain a polar bond but, the molecule may not be polar.
Let us now understand the structure and bonding in both $B{F_3}$ and $N{F_3}$.
The molecule of $B{F_3}$ contains 1 boron atom and 3 fluorine atoms containing 3 lone pairs of electrons on each fluorine atom. The structure of $B{F_3}$ is given below.

Also according to VSEPR, the $B{F_3}$ molecule has trigonal planar geometry and each $F - B - F$ is of 120°. The $B{F_3}$ molecule is highly symmetrical due to its geometry. Also, the dipole moment of a molecule is a vector quantity that means it requires both magnitude and direction. The three bonds in $B{F_3}$ are polar and they have dipole moments as shown below.

The bond dipole moments in $B{F_3}$ are cancelled due to its symmetrical geometry resulting in a net dipole moment of $B{F_3}$ equal to zero. Hence, $B{F_3}$ is non-polar.
The molecule of $N{F_3}$ contains 1 nitrogen atom and 3 fluorine atoms containing 1 lone pair of electrons on nitrogen atom. The structure of $N{F_3}$ is given below.

In the molecule $N{F_3}$, three fluorine atoms one electron each to form covalent bonds with nitrogen atoms and nitrogen atom is left with a lone pair of electrons. The repulsion between lone pair of electrons on nitrogen and bond pairs of electrons $N{F_3}$ molecule leads to bent shape of molecule and the resulting geometry is trigonal pyramidal. Hence, the three dipoles on $N - F$ bond originate towards downward direction and one dipole is in upward direction because of lone pair of electrons. The unsymmetrical geometry of $N{F_3}$ molecule causes a non-zero dipole moment in the molecule.

The molecule $B{F_3}$ and $N{F_3}$ both are covalent compounds but $B{F_3}$ is non-polar and $N{F_3}$ is polar, the reason is that $B{F_3}$ is symmetrical molecule whereas $N{F_3}$ is unsymmetrical.
Hence, the correct answer is option (D).
Note: The compound $N{F_3}$is polar as the sharing of valence electrons between atoms in the compound does not take place equally. On the other hand, $B{F_3}$is a polar compound as electrons are shared equally by atoms in a molecule.
The dipole moment is the product of magnitude of the charge and the distance between the centres of positive and negative charges.
Therefore, mathematically dipole moment can be expressed as,
$Dipole\; Moment = charge \times distance\;separation$
$\mu = \delta \times d$
Here, $\mu $is the dipole moment of a bond, $\delta $ represents the magnitude of partial charges ${\delta ^ + }$ and ${\delta ^ - }$. The letter d represents the distance between partial charges ${\delta ^ + }$ and ${\delta ^ - }$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




