
The molecule with linear structure is:
A) $\text{ Xe}{{\text{O}}_{3}}\text{ }$
B) $\text{ Xe}{{\text{F}}_{\text{4}}}\text{ }$
C) $\text{ Xe}{{\text{F}}_{\text{6}}}\text{ }$
D) $\text{ Xe}{{\text{F}}_{\text{2}}}\text{ }$
Answer
566.7k+ views
Hint: Number of xenon compounds have been synthesised. In $\text{ Xe}{{\text{O}}_{3}}\text{ }$xenon is $\text{ s}{{\text{p}}^{\text{3 }}}$ hybridised, $\text{ Xe}{{\text{F}}_{\text{4}}}\text{ }$ is a \[\text{ s}{{\text{p}}^{\text{3}}}{{\text{d}}^{\text{2}}}\text{ }\] hybridised, $\text{ Xe}{{\text{F}}_{\text{6}}}\text{ }$is a \[\text{ s}{{\text{p}}^{\text{3}}}{{\text{d}}^{3}}\text{ }\]hybridised and $\text{ Xe}{{\text{F}}_{\text{2}}}\text{ }$ is \[\text{ s}{{\text{p}}^{\text{3}}}\text{d }\] hybridised molecules. The xenon difluoride is bound to five electron pairs. There are two bond pairs and three lone pairs.
Complete step by step answer:
Xenon difluoride $\text{ Xe}{{\text{F}}_{\text{2}}}\text{ }$ is a xenon compound. Xenon is a noble gas element. The electronic configuration of xenon is as shown below,
$\text{ Xe = }\left[ \text{Kr} \right]\text{4}{{\text{d}}^{\text{10}}}\text{5}{{\text{s}}^{\text{2}}}\text{5}{{\text{p}}^{\text{6}}}\text{ }$
It has 8 valence electrons. Fluorine atom has an electronic configuration as follows,
$\text{ F = 1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}^{\text{5}}}\text{ }$
Fluorine atoms have 7 valence electrons. In xenon difluoride, each xenon atom gives 8 valence electrons and two fluorine atoms has 14 valence electrons. Thus there are a total of 22 valence electrons in xenon difluoride molecules.
This means that two fluorine atoms are bonded to the xenon atom. Four valence electrons are involved in the formation of two $\text{ Xe}-\text{F }$ bonds. Two fluorine atoms bound to 8 valence electrons of xenon atom. This leaves behind 6 non bonded electron pairs. These 6 electrons are accommodated as 3 lone pairs on the xenon atom.
Xenon atom is surrounded by two bonding pairs and three lone pairs. That is in $\text{ Xe}{{\text{F}}_{\text{2}}}\text{ }$, xenon atom is surrounded by 5 electron pairs .therefore ,xenon difluoride has a trigonal bipyramidal geometry.
To attain the stability, three lone pairs are placed in the equatorial plane and two $\text{ Xe}-\text{F }$ bond pairs are placed perpendicular to the equatorial plane.
Since two fluorine atoms are placed at $\text{ 18}{{\text{0}}^{\text{0}}}\text{ }$ angle .thus xenon difluoride $\text{ Xe}{{\text{F}}_{\text{2}}}\text{ }$ forms a linear geometry. The three lone pairs are arranged symmetrical around the xenon atom. Each lone pair is at the $\text{ 12}{{\text{0}}^{\text{0}}}\text{ }$ angles. The structure of $\text{ Xe}{{\text{F}}_{\text{2}}}\text{ }$ is as shown below,
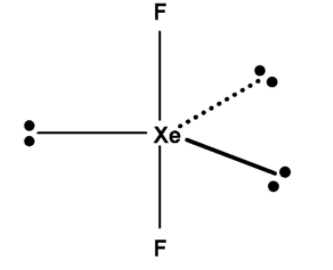
Hence, (D) is the correct option.
Note: Note that, two $\text{ Xe}-\text{F }$ bonds are opposite to each other. Thus net repulsion between the $\text{ Xe}-\text{F }$ bond pair is zero. Thus $\text{ Xe}{{\text{F}}_{\text{2}}}\text{ }$ has linear geometry and has net zero dipole moment.
Complete step by step answer:
Xenon difluoride $\text{ Xe}{{\text{F}}_{\text{2}}}\text{ }$ is a xenon compound. Xenon is a noble gas element. The electronic configuration of xenon is as shown below,
$\text{ Xe = }\left[ \text{Kr} \right]\text{4}{{\text{d}}^{\text{10}}}\text{5}{{\text{s}}^{\text{2}}}\text{5}{{\text{p}}^{\text{6}}}\text{ }$
It has 8 valence electrons. Fluorine atom has an electronic configuration as follows,
$\text{ F = 1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}^{\text{5}}}\text{ }$
Fluorine atoms have 7 valence electrons. In xenon difluoride, each xenon atom gives 8 valence electrons and two fluorine atoms has 14 valence electrons. Thus there are a total of 22 valence electrons in xenon difluoride molecules.
This means that two fluorine atoms are bonded to the xenon atom. Four valence electrons are involved in the formation of two $\text{ Xe}-\text{F }$ bonds. Two fluorine atoms bound to 8 valence electrons of xenon atom. This leaves behind 6 non bonded electron pairs. These 6 electrons are accommodated as 3 lone pairs on the xenon atom.
Xenon atom is surrounded by two bonding pairs and three lone pairs. That is in $\text{ Xe}{{\text{F}}_{\text{2}}}\text{ }$, xenon atom is surrounded by 5 electron pairs .therefore ,xenon difluoride has a trigonal bipyramidal geometry.
To attain the stability, three lone pairs are placed in the equatorial plane and two $\text{ Xe}-\text{F }$ bond pairs are placed perpendicular to the equatorial plane.
Since two fluorine atoms are placed at $\text{ 18}{{\text{0}}^{\text{0}}}\text{ }$ angle .thus xenon difluoride $\text{ Xe}{{\text{F}}_{\text{2}}}\text{ }$ forms a linear geometry. The three lone pairs are arranged symmetrical around the xenon atom. Each lone pair is at the $\text{ 12}{{\text{0}}^{\text{0}}}\text{ }$ angles. The structure of $\text{ Xe}{{\text{F}}_{\text{2}}}\text{ }$ is as shown below,

Hence, (D) is the correct option.
Note: Note that, two $\text{ Xe}-\text{F }$ bonds are opposite to each other. Thus net repulsion between the $\text{ Xe}-\text{F }$ bond pair is zero. Thus $\text{ Xe}{{\text{F}}_{\text{2}}}\text{ }$ has linear geometry and has net zero dipole moment.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




