
The monomer of terylene are:
A. Phenol and formaldehyde
B. Ethylene glycol and phthalic acid
C. Adipic acid and hexamethylenediamine
D. Ethylene glycol and terephthalic acid
Answer
584.7k+ views
Hint: The structure of terylene consists of 1,4- dicarboxylic acid and ethane-1,2-diol. Dacron is the other name for terylene. Due to the presence of ester linkage, it is also known as polyester.
Complete Step-by-Step Answer:
-The structure of terylene is:
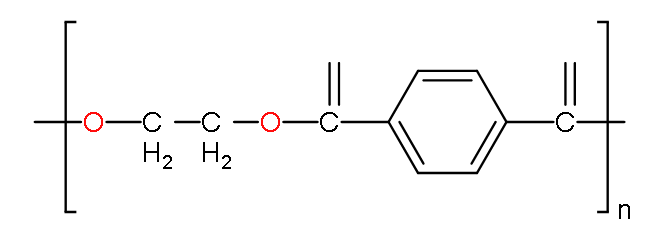
-In terylene, two main molecules bond together as a monomer by the process of polymerisation to form a polymer known as terylene.
-The monomers which polymerise are ethylene glycol and terephthalic acid.
-The molecular formula of ethylene glycol is $\text{nHO}{{\text{H}}_{2}}\text{C-C}{{\text{H}}_{2}}\text{OH}$
-The molecular formula of phthalic acid is \[\text{nHOOC-}{{\text{C}}_{6}}{{\text{H}}_{5}}\text{-COOH}\]
-The reaction of the formation of terylene is:

-They both react with each other and forms:
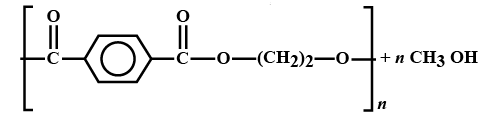
-So, option D. is an correct answer.
-Phenol and formaldehyde are the monomers which on polymerisation form Bakelite.
-So, option A. is an incorrect answer.
-Ethylene glycol and phthalic acid are the monomers which on polymerisation form Glyptal.
-So, option B. is also an incorrect answer.
-Adipic and hexamethylenediamine are the monomers which form polymerisation form Nylon-6,6.
-So, option C. is also an incorrect answer.
Therefore, option D. is the correct answer.
Note: The difference between polymer and monomers is that monomer is the smallest and repeating unit which bonds together and makes a long chain which is known as a polymer. For example, polythene is a long-chain polymer which is made from the ethene. Many molecules of ethene combine and make bonds to form a long chain of polythene. That’s why it is named as 'poly' which means 'many'.
Complete Step-by-Step Answer:
-The structure of terylene is:

-In terylene, two main molecules bond together as a monomer by the process of polymerisation to form a polymer known as terylene.
-The monomers which polymerise are ethylene glycol and terephthalic acid.
-The molecular formula of ethylene glycol is $\text{nHO}{{\text{H}}_{2}}\text{C-C}{{\text{H}}_{2}}\text{OH}$
-The molecular formula of phthalic acid is \[\text{nHOOC-}{{\text{C}}_{6}}{{\text{H}}_{5}}\text{-COOH}\]
-The reaction of the formation of terylene is:

-They both react with each other and forms:
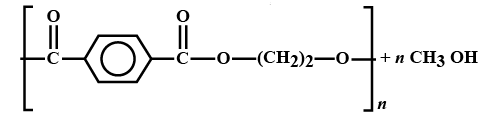
-So, option D. is an correct answer.
-Phenol and formaldehyde are the monomers which on polymerisation form Bakelite.
-So, option A. is an incorrect answer.
-Ethylene glycol and phthalic acid are the monomers which on polymerisation form Glyptal.
-So, option B. is also an incorrect answer.
-Adipic and hexamethylenediamine are the monomers which form polymerisation form Nylon-6,6.
-So, option C. is also an incorrect answer.
Therefore, option D. is the correct answer.
Note: The difference between polymer and monomers is that monomer is the smallest and repeating unit which bonds together and makes a long chain which is known as a polymer. For example, polythene is a long-chain polymer which is made from the ethene. Many molecules of ethene combine and make bonds to form a long chain of polythene. That’s why it is named as 'poly' which means 'many'.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




