
The nitrogen containing compound produced in the reaction of $HN{O_3}$ with ${P_4}{O_{10}}$ is:
A. can also be prepared by reaction of ${P_4}$and $HN{O_3}$
B. is diamagnetic
C. contains one $N - N$ bond
D. reacts with $Na$ metal producing a brown gas.
Answer
582.9k+ views
Hint: The nitrogen containing compound produced in the reactions of $HN{O_3}$ with ${P_4}{O_{10}}$ is ${N_2}{O_5}.$ . The name of the compound formed is dinitrogen pentoxide and it is one of the members of the family of compounds that contain only nitrogen and oxygen.
Complete step by step answer: The reaction of $HN{O_3}$ and ${P_4}{O_{10}}$ result in the production of nitrogen containing compound ${N_2}{O_5}$
The reaction involved is :
$4HN{O_3} + {P_4}{O_{10}} \to 2{N_2}{O_5} + 4HP{O_3}$
In the option A,
${P_4} + 20HN{O_3} \to 4{H_3}P{O_4} + 20N{O_2} + 4{H_2}O$
So, with ${P_4}$ and $HN{O_3}$ we cannot produce the some nitrogen compound that was prepared with ${P_4}{O_{10}}$ and $HN{O_3}$
In option B, it is said that the compound ${N_2}{O_5}.$ produced is diamagnetic in nature as it does not contain any unpaired electron.
For option C, it explains that there is One $N - N$ bond in ${N_2}{O_5}$ but no there is no such bond. The structure of ${N_2}{O_5}$ is :
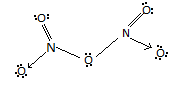
For option D, it reveals that the reaction of ${N_2}{O_5}$ with $Na$ metal produces a brown gas. So, the reaction of ${N_2}{O_5}$ with $Na$ produces $N{O_2}$ which is a reddish – brown gas. Hence, this option is also there.
The reaction is :
${N_2}{O_5} + Na \to NaN{O_3} + N{O_2}$ .
Therefore, the options B and D are the correct one.
Additional Information: Dinitrogen pentoxide is an unstable and dangerous oxidizer. it generally exists as colourless crystals and sublimes at temperature slightly above room temperature yielding a colorless gas. It is a compound that adopts two structures depending upon the conditions. In a solid state it is a salt, nitronium nitrate and in the gaseous phase it is a covalently bonded molecule.
Note: The compound formed that is dinitrogen pentoxide is also known as nitric anhydride. It is a strong oxidizer that produces explosive mixtures with organic compounds and ammonium salts. It produces highly toxic gas nitrogen dioxide on decomposition.
Complete step by step answer: The reaction of $HN{O_3}$ and ${P_4}{O_{10}}$ result in the production of nitrogen containing compound ${N_2}{O_5}$
The reaction involved is :
$4HN{O_3} + {P_4}{O_{10}} \to 2{N_2}{O_5} + 4HP{O_3}$
In the option A,
${P_4} + 20HN{O_3} \to 4{H_3}P{O_4} + 20N{O_2} + 4{H_2}O$
So, with ${P_4}$ and $HN{O_3}$ we cannot produce the some nitrogen compound that was prepared with ${P_4}{O_{10}}$ and $HN{O_3}$
In option B, it is said that the compound ${N_2}{O_5}.$ produced is diamagnetic in nature as it does not contain any unpaired electron.
For option C, it explains that there is One $N - N$ bond in ${N_2}{O_5}$ but no there is no such bond. The structure of ${N_2}{O_5}$ is :

For option D, it reveals that the reaction of ${N_2}{O_5}$ with $Na$ metal produces a brown gas. So, the reaction of ${N_2}{O_5}$ with $Na$ produces $N{O_2}$ which is a reddish – brown gas. Hence, this option is also there.
The reaction is :
${N_2}{O_5} + Na \to NaN{O_3} + N{O_2}$ .
Therefore, the options B and D are the correct one.
Additional Information: Dinitrogen pentoxide is an unstable and dangerous oxidizer. it generally exists as colourless crystals and sublimes at temperature slightly above room temperature yielding a colorless gas. It is a compound that adopts two structures depending upon the conditions. In a solid state it is a salt, nitronium nitrate and in the gaseous phase it is a covalently bonded molecule.
Note: The compound formed that is dinitrogen pentoxide is also known as nitric anhydride. It is a strong oxidizer that produces explosive mixtures with organic compounds and ammonium salts. It produces highly toxic gas nitrogen dioxide on decomposition.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




