
The number of \[{{1}^{o}}\], \[{{2}^{o}}\], and \[{{3}^{o}}\] H atoms in 2,5,6 – trimethyl octane, respectively, is
(A) 16,5,3
(B) 15,6,3
(C) 16,6,3
(D) 15,5,2
Answer
569.7k+ views
Hint: The primary hydrogen atom is the atom which is attached to only one carbon atom. Generally the group attached to the primary hydrogen will be methyl group.
The secondary hydrogen atom is the atom which is attached to two other carbon atoms. The group attached to secondary hydrogen will be \[C{{H}_{2}}\] group
The tertiary hydrogen atom is the atom which is attached to three other carbon atoms. The group attached to tertiary hydrogen will be the \[CH\] group.
Complete step by step solution:
In the 2,5,6 trimethyl octane the methyl group is attached to 2,5,6 positioned carbon atoms.
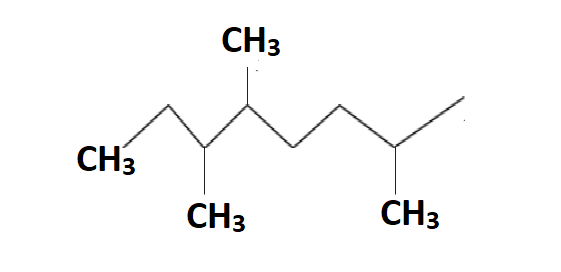
In the above structure of 2,5,6 trimethyl octane we can see the methyl groups, hydrogen groups position and carbon atoms clearly.
So by the definition of primary hydrogen atom, in this structure we can see 15 hydrogen atoms which are attached to only one carbon atom which is the hydrogen attached to the methyl group.
So by the definition of secondary hydrogen atom, in this structure we can see 6 hydrogen atoms which are attached to two carbon atoms which are the hydrogen attached to \[C{{H}_{2}}\] group.
So by the definition of tertiary hydrogen atom, in this structure we can see 3 hydrogen atoms which are attached to three carbon atoms which are the hydrogen attached to the \[CH\] group.
Hence, the correct option for this question is option (B).
Note: Students should know the structure of all the organic compounds. Students should make the structure of the compounds to gain marks. Do write all the definitions of all primary, secondary and tertiary hydrogen atoms to show the teacher that your concept is clear. In this structure we have started the counting of carbon atoms from right, but in other questions of such type you need to start the counting from functional groups only to get the correct number of hydrogen atoms. The different functional groups are alcohols, halogens , aldehydes, nitrates,etc.
The secondary hydrogen atom is the atom which is attached to two other carbon atoms. The group attached to secondary hydrogen will be \[C{{H}_{2}}\] group
The tertiary hydrogen atom is the atom which is attached to three other carbon atoms. The group attached to tertiary hydrogen will be the \[CH\] group.
Complete step by step solution:
In the 2,5,6 trimethyl octane the methyl group is attached to 2,5,6 positioned carbon atoms.

In the above structure of 2,5,6 trimethyl octane we can see the methyl groups, hydrogen groups position and carbon atoms clearly.
So by the definition of primary hydrogen atom, in this structure we can see 15 hydrogen atoms which are attached to only one carbon atom which is the hydrogen attached to the methyl group.
So by the definition of secondary hydrogen atom, in this structure we can see 6 hydrogen atoms which are attached to two carbon atoms which are the hydrogen attached to \[C{{H}_{2}}\] group.
So by the definition of tertiary hydrogen atom, in this structure we can see 3 hydrogen atoms which are attached to three carbon atoms which are the hydrogen attached to the \[CH\] group.
Hence, the correct option for this question is option (B).
Note: Students should know the structure of all the organic compounds. Students should make the structure of the compounds to gain marks. Do write all the definitions of all primary, secondary and tertiary hydrogen atoms to show the teacher that your concept is clear. In this structure we have started the counting of carbon atoms from right, but in other questions of such type you need to start the counting from functional groups only to get the correct number of hydrogen atoms. The different functional groups are alcohols, halogens , aldehydes, nitrates,etc.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

What is the difference between biodegradable and nonbiodegradable class 11 biology CBSE

Proton was discovered by A Thomson B Rutherford C Chadwick class 11 chemistry CBSE




