
The number of σ bonds and π bonds in methyl cyanide are x and y respectively. Value of $x + y$ is
Answer
578.1k+ views
Hint: Methyl cyanide is also known as acetonitrile and is the simplest organic nitrile. It is used as a polar aprotic solvent in organic synthesis and also used in the purification of butadiene.
Complete step by step answer:
To find out the number of sigmas and pi bonds in the case of the given compound first we will write the complete structural formula of the compound then count the number of carbon-carbon, carbon-hydrogen, and carbon-nitrogen sigma bond and carbon-nitrogen pi bond.
Since the formula of methyl cyanide is given as:
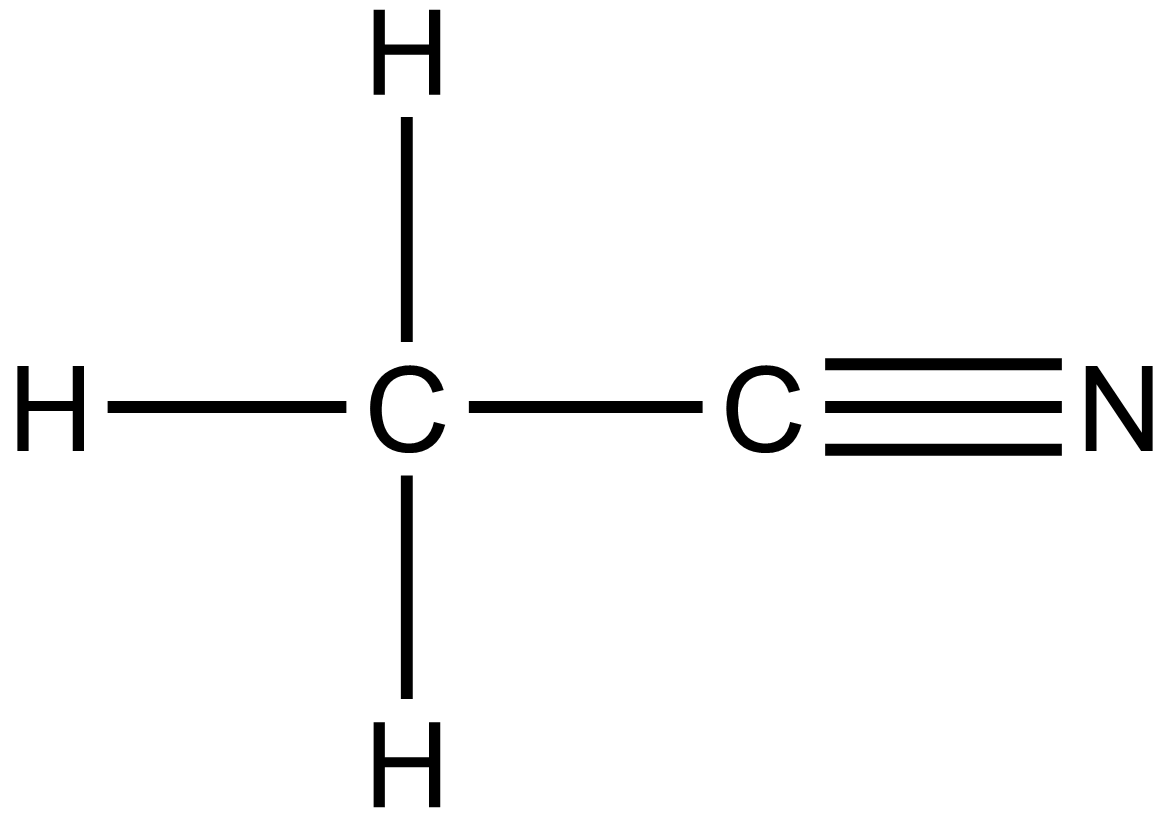
Here 5 sigma bonds are present since x in the formula represents the number of sigma bonds thus x=5 and there are two pi bonds present. In this question pi bond is presented by y, thus y =2.
And the sum of x and y will be
$x + y = 5 + 2 = 7$
Additional information: Sigma and pi bonds are the types of a covalent bond. The classification of the covalent bond into these two types is based on overlapping. Sigma bond is defined as the bond which is formed by the overlapping of atomic orbitals on the internuclear axis whereas the pi bonds are defined as the bonds which are formed by the lateral or sideways overlapping of the p orbitals. Formation of sigma bond involves three types of overlapping such as s-s overlapping, s-p overlapping, and p-p overlapping but in the case of formation of pi bond, there is only one type of overlapping .i.e. p-p overlapping.
Note:
Sigma bond is stronger whereas the pi bonds are weaker because in case sigma bond overlapping is along the internuclear axis whereas, in case of the pi bond, overlapping is either above the plane or below the plane.
Complete step by step answer:
To find out the number of sigmas and pi bonds in the case of the given compound first we will write the complete structural formula of the compound then count the number of carbon-carbon, carbon-hydrogen, and carbon-nitrogen sigma bond and carbon-nitrogen pi bond.
Since the formula of methyl cyanide is given as:

Here 5 sigma bonds are present since x in the formula represents the number of sigma bonds thus x=5 and there are two pi bonds present. In this question pi bond is presented by y, thus y =2.
And the sum of x and y will be
$x + y = 5 + 2 = 7$
Additional information: Sigma and pi bonds are the types of a covalent bond. The classification of the covalent bond into these two types is based on overlapping. Sigma bond is defined as the bond which is formed by the overlapping of atomic orbitals on the internuclear axis whereas the pi bonds are defined as the bonds which are formed by the lateral or sideways overlapping of the p orbitals. Formation of sigma bond involves three types of overlapping such as s-s overlapping, s-p overlapping, and p-p overlapping but in the case of formation of pi bond, there is only one type of overlapping .i.e. p-p overlapping.
Note:
Sigma bond is stronger whereas the pi bonds are weaker because in case sigma bond overlapping is along the internuclear axis whereas, in case of the pi bond, overlapping is either above the plane or below the plane.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




